Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
5-hydroxytryptamine receptor 2B
Ligand
BDBM50130269
Substrate
n/a
Ki
12.6±n/a nM
Comments
PDSP_1095
Citation
 Wainscott, DB; Lucaites, VL; Kursar, JD; Baez, M; Nelson, DL Pharmacologic characterization of the human 5-hydroxytryptamine2B receptor: evidence for species differences. J Pharmacol Exp Ther 276:720-7 (1996) [PubMed]
Wainscott, DB; Lucaites, VL; Kursar, JD; Baez, M; Nelson, DL Pharmacologic characterization of the human 5-hydroxytryptamine2B receptor: evidence for species differences. J Pharmacol Exp Ther 276:720-7 (1996) [PubMed] More Info.:
Target
Name:
5-hydroxytryptamine receptor 2B
Synonyms:
5-HT2B | 5-hydroxytryptamine receptor 2B | 5HT2B_RAT | Htr2b | Serotonin 2b (5-HT2b) receptor | Serotonin receptor 2a and 2b (5HT2A and 5HT2B) | Srl
Type:
Enzyme Catalytic Domain
Mol. Mass.:
53668.47
Organism:
RAT
Description:
5-HT2B HTR2B RAT::P30994
Residue:
479
Sequence:
MASSYKMSEQSTISEHILQKTCDHLILTDRSGLKAESAAEEMKQTAENQGNTVHWAALLIFAVIIPTIGGNILVILAVSLEKRLQYATNYFLMSLAVADLLVGLFVMPIALLTIMFEATWPLPLALCPAWLFLDVLFSTASIMHLCAISLDRYIAIKKPIQANQCNSRTTAFVKITVVWLISIGIAIPVPIKGIEADVVNAHNITCELTKDRFGSFMLFGSLAAFFAPLTIMIVTYFLTIHALRKKAYLVRNRPPQRLTRWTVSTVLQREDSSFSSPEKMVMLDGSHKDKILPNSTDETLMRRMSSAGKKPAQTISNEQRASKVLGIVFLFFLLMWCPFFITNVTLALCDSCNQTTLKTLLQIFVWVGYVSSGVNPLIYTLFNKTFREAFGRYITCNYQATKSVKVLRKCSSTLYFGNSMVENSKFFTKHGIRNGINPAMYQSPVRLRSSTIQSSSIILLNTFLTENDGDKVEDQVSYI
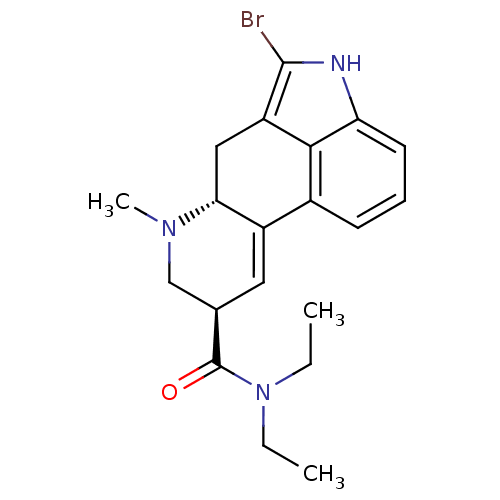
Inhibitor
Name:
BDBM50130269
Synonyms:
(6aR,9R)-5-Bromo-7-methyl-4,6,6a,7,8,9-hexahydro-indolo[4,3-fg]quinoline-9-carboxylic acid diethylamide | 5-Bromo-7-methyl-4,6,6a,7,8,9-hexahydro-indolo[4,3-fg]quinoline-9-carboxylic acid diethylamide | CHEMBL274384 | LSD,2-Bromo
Type:
Small organic molecule
Emp. Form.:
C20H24BrN3O
Mol. Mass.:
402.328
SMILES:
CCN(CC)C(=O)[C@H]1CN(C)[C@@H]2Cc3c(Br)[nH]c4cccc(C2=C1)c34 |c:23|