Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Oxysterols receptor LXR-beta
Ligand
BDBM115112
Substrate
n/a
Meas. Tech.
Counterscreen for agonists of the daf-12 abnormal Dauer Formation: Luminescence-based cell-based dose response screening assay to identify agonists of the Liver-X-Receptor (LXR)
EC50
67535±n/a nM
Citation
 PubChem, PC Counterscreen for agonists of the daf-12 abnormal Dauer Formation: Luminescence-based cell-based dose response screening assay to identify agonists of the Liver-X-Receptor (LXR) PubChem Bioassay (2013)[AID]
PubChem, PC Counterscreen for agonists of the daf-12 abnormal Dauer Formation: Luminescence-based cell-based dose response screening assay to identify agonists of the Liver-X-Receptor (LXR) PubChem Bioassay (2013)[AID] More Info.:
Target
Name:
Oxysterols receptor LXR-beta
Synonyms:
LXRB | Liver X receptor beta (NR1H2) | Liver X, LXR beta | NER | NR1H2 | NR1H2_HUMAN | Nuclear receptor NER | UNR | Ubiquitously-expressed nuclear receptor
Type:
Enzyme Catalytic Domain
Mol. Mass.:
50978.79
Organism:
Homo sapiens (Human)
Description:
P55055
Residue:
460
Sequence:
MSSPTTSSLDTPLPGNGPPQPGAPSSSPTVKEEGPEPWPGGPDPDVPGTDEASSACSTDWVIPDPEEEPERKRKKGPAPKMLGHELCRVCGDKASGFHYNVLSCEGCKGFFRRSVVRGGARRYACRGGGTCQMDAFMRRKCQQCRLRKCKEAGMREQCVLSEEQIRKKKIRKQQQESQSQSQSPVGPQGSSSSASGPGASPGGSEAGSQGSGEGEGVQLTAAQELMIQQLVAAQLQCNKRSFSDQPKVTPWPLGADPQSRDARQQRFAHFTELAIISVQEIVDFAKQVPGFLQLGREDQIALLKASTIEIMLLETARRYNHETECITFLKDFTYSKDDFHRAGLQVEFINPIFEFSRAMRRLGLDDAEYALLIAINIFSADRPNVQEPGRVEALQQPYVEALLSYTRIKRPQDQLRFPRMLMKLVSLRTLSSVHSEQVFALRLQDKKLPPLLSEIWDVHE
Inhibitor
Name:
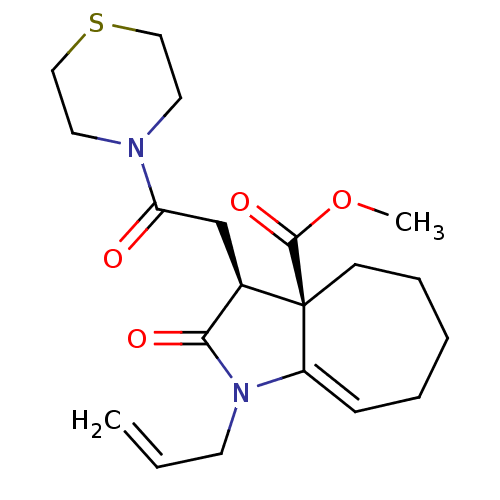
BDBM115112
Synonyms:
(3S,3aR)-1-allyl-2-keto-3-(2-keto-2-thiomorpholino-ethyl)-4,5,6,7-tetrahydro-3H-cyclohepta[b]pyrrole-3a-carboxylic acid methyl ester | (3S,3aR)-2-oxo-3-(2-oxo-2-thiomorpholin-4-ylethyl)-1-prop-2-enyl-4,5,6,7-tetrahydro-3H-cyclohepta[b]pyrrole-3a-carboxylic acid methyl ester | MLS004259454 | SMR003089419 | cid_60158113 | methyl (3S,3aR)-2-oxidanylidene-3-(2-oxidanylidene-2-thiomorpholin-4-yl-ethyl)-1-prop-2-enyl-4,5,6,7-tetrahydro-3H-cyclohepta[b]pyrrole-3a-carboxylate | methyl (3S,3aR)-2-oxo-3-(2-oxo-2-thiomorpholin-4-ylethyl)-1-prop-2-enyl-4,5,6,7-tetrahydro-3H-cyclohepta[b]pyrrole-3a-carboxylate
Type:
Small organic molecule
Emp. Form.:
C20H28N2O4S
Mol. Mass.:
392.512
SMILES:
COC(=O)[C@@]12CCCCC=C1N(CC=C)C(=O)[C@H]2CC(=O)N1CCSCC1 |c:9|