Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
DNA topoisomerase 2-alpha
Ligand
BDBM50127140
Substrate
n/a
Meas. Tech.
Topoisomerase IIα Relaxation Assay
pH
7.9±n/a
IC50
5.954e+4±n/a nM
Comments
extracted
Citation
More Info.:
Target
Name:
DNA topoisomerase 2-alpha
Synonyms:
DNA topoisomerase 2-alpha | DNA topoisomerase II | DNA topoisomerase II (Topo II) | DNA topoisomerase II alpha | DNA topoisomerase II, alpha isozyme | TOP2 | TOP2A | TOP2A_HUMAN | Topoisomerase I/II | Topoisomerase II alpha (HuTopoIIα)
Type:
Protein
Mol. Mass.:
174415.30
Organism:
Homo sapiens (Human)
Description:
P11388
Residue:
1531
Sequence:
MEVSPLQPVNENMQVNKIKKNEDAKKRLSVERIYQKKTQLEHILLRPDTYIGSVELVTQQMWVYDEDVGINYREVTFVPGLYKIFDEILVNAADNKQRDPKMSCIRVTIDPENNLISIWNNGKGIPVVEHKVEKMYVPALIFGQLLTSSNYDDDEKKVTGGRNGYGAKLCNIFSTKFTVETASREYKKMFKQTWMDNMGRAGEMELKPFNGEDYTCITFQPDLSKFKMQSLDKDIVALMVRRAYDIAGSTKDVKVFLNGNKLPVKGFRSYVDMYLKDKLDETGNSLKVIHEQVNHRWEVCLTMSEKGFQQISFVNSIATSKGGRHVDYVADQIVTKLVDVVKKKNKGGVAVKAHQVKNHMWIFVNALIENPTFDSQTKENMTLQPKSFGSTCQLSEKFIKAAIGCGIVESILNWVKFKAQVQLNKKCSAVKHNRIKGIPKLDDANDAGGRNSTECTLILTEGDSAKTLAVSGLGVVGRDKYGVFPLRGKILNVREASHKQIMENAEINNIIKIVGLQYKKNYEDEDSLKTLRYGKIMIMTDQDQDGSHIKGLLINFIHHNWPSLLRHRFLEEFITPIVKVSKNKQEMAFYSLPEFEEWKSSTPNHKKWKVKYYKGLGTSTSKEAKEYFADMKRHRIQFKYSGPEDDAAISLAFSKKQIDDRKEWLTNFMEDRRQRKLLGLPEDYLYGQTTTYLTYNDFINKELILFSNSDNERSIPSMVDGLKPGQRKVLFTCFKRNDKREVKVAQLAGSVAEMSSYHHGEMSLMMTIINLAQNFVGSNNLNLLQPIGQFGTRLHGGKDSASPRYIFTMLSSLARLLFPPKDDHTLKFLYDDNQRVEPEWYIPIIPMVLINGAEGIGTGWSCKIPNFDVREIVNNIRRLMDGEEPLPMLPSYKNFKGTIEELAPNQYVISGEVAILNSTTIEISELPVRTWTQTYKEQVLEPMLNGTEKTPPLITDYREYHTDTTVKFVVKMTEEKLAEAERVGLHKVFKLQTSLTCNSMVLFDHVGCLKKYDTVLDILRDFFELRLKYYGLRKEWLLGMLGAESAKLNNQARFILEKIDGKIIIENKPKKELIKVLIQRGYDSDPVKAWKEAQQKVPDEEENEESDNEKETEKSDSVTDSGPTFNYLLDMPLWYLTKEKKDELCRLRNEKEQELDTLKRKSPSDLWKEDLATFIEELEAVEAKEKQDEQVGLPGKGGKAKGKKTQMAEVLPSPRGQRVIPRITIEMKAEAEKKNKKKIKNENTEGSPQEDGVELEGLKQRLEKKQKREPGTKTKKQTTLAFKPIKKGKKRNPWSDSESDRSSDESNFDVPPRETEPRRAATKTKFTMDLDSDEDFSDFDEKTDDEDFVPSDASPPKTKTSPKLSNKELKPQKSVVSDLEADDVKGSVPLSSSPPATHFPDETEITNPVPKKNVTVKKTAAKSQSSTSTTGAKKRAAPKGTKRDPALNSGVSQKPDPAKTKNRRKRKPSTSDDSDSNFEKIVSKAVTSKKSKGESDDFHMDFDSAVAPRAKSVRAKKPIKYLEESDEDDLF
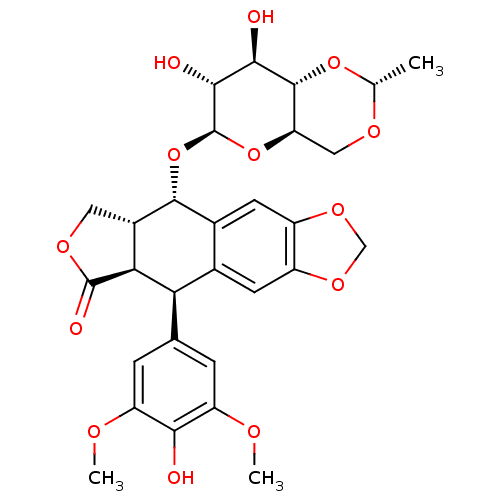
Inhibitor
Name:
BDBM50127140
Synonyms:
(-)-etoposide | (5S,5aR,8aR,9R)-9-(4-hydroxy-3,5-dimethoxyphenyl)-8-oxo-5,5a,6,8,8a,9-hexahydrofuro[3',4':6,7]naphtho[2,3-d][1,3]dioxol-5-yl 4,6-O-[(1R)-ethylidene]-beta-D-glucopyranoside | 4'-Demethylepipodophyllotoxin 9-(4,6-O-(R)-ethylidene-beta-D-glucopyranoside) | 4-demethylepipodophyllotoxin beta-D-ethylideneglucoside | 9-((4,6-O-Ethylidine-beta-D-glucopyranosyl)oxy)-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,4-dimethyloxyphenyl)furo(3',4'':6,7)naptho-(2,3-d)-1,3-dioxol-6(5aH)-one | CHEMBL44657 | Etoposide (ET) | TRANS-ETOPOSIDE | VP-16 | etoposide
Type:
Small organic molecule
Emp. Form.:
C29H32O13
Mol. Mass.:
588.5566
SMILES:
COc1cc(cc(OC)c1O)[C@H]1[C@@H]2[C@H](COC2=O)[C@H](O[C@@H]2O[C@@H]3CO[C@@H](C)O[C@H]3[C@H](O)[C@H]2O)c2cc3OCOc3cc12 |r|