Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Lysosomal alpha-glucosidase
Ligand
BDBM23406
Substrate
n/a
Meas. Tech.
Inhibition Assay
pH
7±n/a
IC50
1.364e+4±n/a nM
Comments
extracted
Citation
 Yousefi, R; Alavian-Mehr, MM; Mokhtari, F; Panahi, F; Mehraban, MH; Khalafi-Nezhad, A Pyrimidine-fused heterocycle derivatives as a novel class of inhibitors for a-glucosidase. J Enzyme Inhib Med Chem 28:1228-35 (2013) [PubMed] Article
Yousefi, R; Alavian-Mehr, MM; Mokhtari, F; Panahi, F; Mehraban, MH; Khalafi-Nezhad, A Pyrimidine-fused heterocycle derivatives as a novel class of inhibitors for a-glucosidase. J Enzyme Inhib Med Chem 28:1228-35 (2013) [PubMed] Article More Info.:
Target
Name:
Lysosomal alpha-glucosidase
Synonyms:
α-Glucosidase | Acid maltase | Gaa | LYAG_MOUSE | Lysosomal alpha-glucosidase | alpha-glucosidase (Gaa)
Type:
Enzyme
Mol. Mass.:
106235.32
Organism:
Mus musculus (Mouse)
Description:
P70699
Residue:
953
Sequence:
MNIRKPLCSNSVVGACTLISLTTAVILGHLMLRELMLLPQDLHESSSGLWKTYRPHHQEGYKPGPLHIQEQTEQPKEAPTQCDVPPSSRFDCAPDKGISQEQCEARGCCYVPAGQVLKEPQIGQPWCFFPPSYPSYRLENLSSTESGYTATLTRTSPTFFPKDVLTLQLEVLMETDSRLHFKIKDPASKRYEVPLETPRVLSQAPSPLYSVEFSEEPFGVIVRRKLGGRVLLNTTVAPLFFADQFLQLSTSLPSQHITGLGEHLSPLMLSTDWARITLWNRDTPPSQGTNLYGSHPFYLALEDGGLAHGVFLLNSNAMDVILQPSPALTWRSTGGILDVYVFLGPEPKSVVQQYLDVVGYPFMPPYWGLGFHLCRWGYSSTAIVRQVVENMTRTHFPLDVQWNDLDYMDARRDFTFNQDSFADFPDMVRELHQDGRRYMMIVDPAISSAGPAGSYRPYDEGLRRGVFITNETGQPLIGKVWPGTTAFPDFTNPETLDWWQDMVSEFHAQVPFDGMWLDMNEPSNFVRGSQQGCPNNELENPPYVPGVVGGILQAATICASSHQFLSTHYNLHNLYGLTEAIASSRALVKTRGTRPFVISRSTFSGHGRYAGHWTGDVRSSWEHLAYSVPDILQFNLLGVPLVGADICGFIGDTSEELCVRWTQLGAFYPFMRNHNDLNSVPQEPYRFSETAQQAMRKAFALRYALLPYLYTLFHRAHVRGDTVARPLFLEFPEDPSTWSVDRQLLWGPALLITPVLEPGKTEVTGYFPKGTWYNMQMVSVDSLGTLPSPSSASSFRSAVQSKGQWLTLEAPLDTINVHLREGYIIPLQGPSLTTTESRKQPMALAVALTASGEADGELFWDDGESLAVLERGAYTLVTFSAKNNTIVNKLVRVTKEGAELQLREVTVLGVATAPTQVLSNGIPVSNFTYSPDNKSLAIPVSLLMGELFQISWS
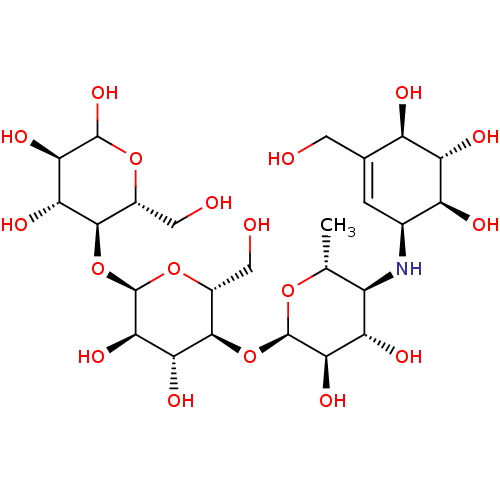
Inhibitor
Name:
BDBM23406
Synonyms:
(3R,4R,5S,6R)-5-{[(2R,3R,4R,5S,6R)-5-{[(2R,3R,4S,5S,6R)-3,4-dihydroxy-6-methyl-5-{[(1S,4R,5S,6S)-4,5,6-trihydroxy-3-(hydroxymethyl)cyclohex-2-en-1-yl]amino}oxan-2-yl]oxy}-3,4-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-6-(hydroxymethyl)oxane-2,3,4-triol | Acarbose | US11292789, Acarbose
Type:
Carbohydrate
Emp. Form.:
C25H43NO18
Mol. Mass.:
645.6048
SMILES:
C[C@H]1O[C@H](O[C@@H]2[C@@H](CO)O[C@H](O[C@@H]3[C@@H](CO)OC(O)[C@H](O)[C@H]3O)[C@H](O)[C@H]2O)[C@H](O)[C@@H](O)[C@@H]1N[C@H]1C=C(CO)[C@@H](O)[C@H](O)[C@H]1O |t:37|