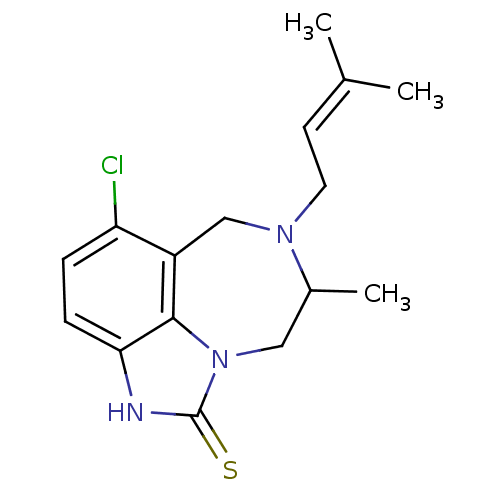
TargetReverse transcriptase/RNaseH(Human immunodeficiency virus type 1)
Ibm Thomas J. Watson Research Center
Curated by ChEMBL
Ibm Thomas J. Watson Research Center
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibitory concentration against HIV-1 replication by interfering with virus reverse transcriptaseMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)