BDBM50446481 CHEMBL3110004::US10011611, TMP269::US10722597, Compound TMP-269
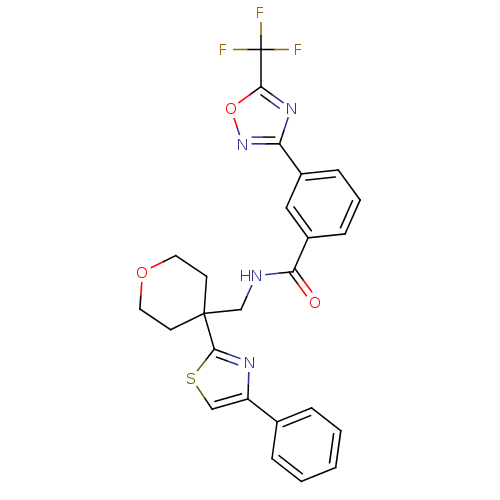
SMILES FC(F)(F)c1nc(no1)-c1cccc(c1)C(=O)NCC1(CCOCC1)c1nc(cs1)-c1ccccc1
InChI Key InChIKey=HORXBWNTEDOVKN-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50446481
Found 4 hits for monomerid = 50446481
Affinity DataIC50: 376nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
Affinity DataIC50: 354nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
Affinity DataIC50: 153nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
Affinity DataIC50: 43.3nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)