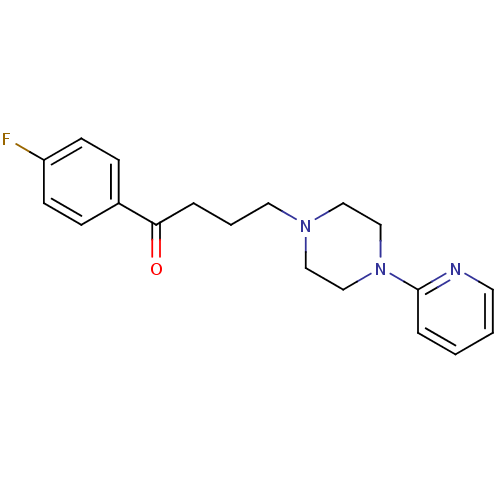
BDBM50036733 1-(4-Fluoro-phenyl)-4-(4-pyridin-2-yl-piperazin-1-yl)-butan-1-one(Azaperone)::Azaperone::CHEMBL340211
SMILES Fc1ccc(cc1)C(=O)CCCN1CCN(CC1)c1ccccn1
InChI Key InChIKey=XTKDAFGWCDAMPY-UHFFFAOYSA-N
Data 38 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50036733
Found 4 hits for monomerid = 50036733
Affinity DataKi: 62.7nMAssay Description:Displacement of [3H]-ketanserin from human 5HT2A receptor by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 62.7nMAssay Description:Displacement of [3H]-Ketanserin from human 5-HT2A receptor incubated for 1 hr by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 63nMAssay Description:Displacement of [3H]-Ketanserin from human 5-HT2A receptor incubated for 1 hr by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 63nMAssay Description:Displacement of [3H]-ketanserin from human 5HT2A receptor by liquid scintillation countingMore data for this Ligand-Target Pair