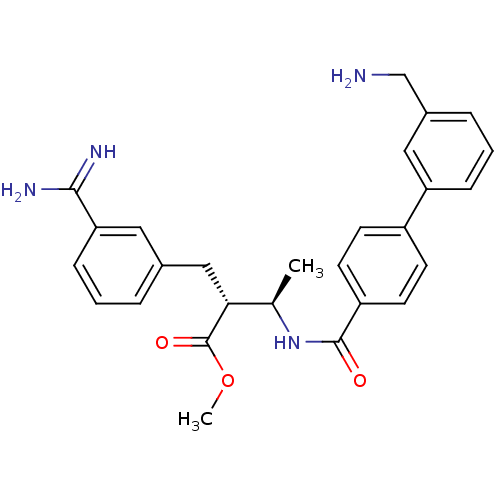
BDBM12597 CHEMBL48046::RPR128515::methyl (2R,3R)-2-{3-[amino(imino)methyl]benzyl}-3-({[3-(aminomethyl)-1,1-biphenyl-4-yl]carbonyl}amino)butanoate::methyl (2R,3R)-3-({4-[3-(aminomethyl)phenyl]phenyl}formamido)-2-[(3-carbamimidoylphenyl)methyl]butanoate
SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cccc(CN)c1
InChI Key InChIKey=XFKVLKLCLYJKNF-MZNJEOGPSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 12597
Found 8 hits for monomerid = 12597
Affinity DataKi: 0.900nM ΔG°: -12.2kcal/molepH: 7.5 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 0.900nM ΔG°: -12.2kcal/molepH: 7.5 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 0.900nMAssay Description:In vitro inhibitory activity against human Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 69nMAssay Description:In vitro inhibitory activity against human trypsinMore data for this Ligand-Target Pair
Affinity DataKi: 69nM ΔG°: -9.66kcal/molepH: 7.5 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 69nM ΔG°: -9.66kcal/molepH: 7.5 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: >3.00E+3nM ΔG°: >-7.45kcal/molepH: 7.5 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: >3.00E+3nM ΔG°: >-7.45kcal/molepH: 7.5 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)