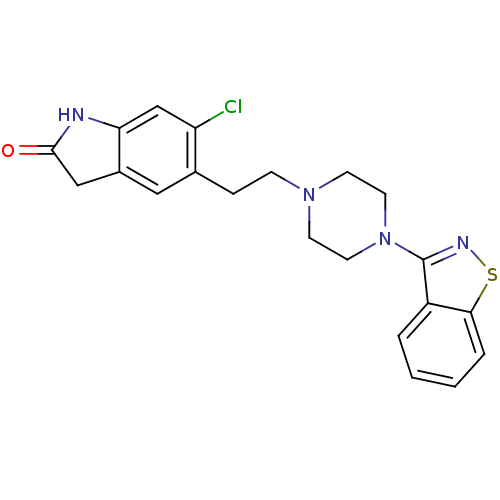
BDBM50048803 5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)ethyl)-6-chloroindolin-2-one::5-[2-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-ethyl]-6-chloro-1,3-dihydro-indol-2-one::5-[2-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-ethyl]-6-chloro-1,3-dihydro-indol-2-one (Ziprasidone)::5-[2-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-ethyl]-6-chloro-1,3-dihydro-indol-2-one(Norastemizole)::CHEMBL708::GEODON::ZIPRASIDONE::ZIPRASIDONE HYDROCHLORIDE
SMILES Clc1cc2NC(=O)Cc2cc1CCN1CCN(CC1)c1nsc2ccccc12
InChI Key InChIKey=MVWVFYHBGMAFLY-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50048803
Found 9 hits for monomerid = 50048803
Target5-hydroxytryptamine receptor 2A(Homo sapiens (Human))
Merck Sharp And Dohme Research Laboratories
Curated by ChEMBL
Merck Sharp And Dohme Research Laboratories
Curated by ChEMBL
Affinity DataKi: 0.25nMAssay Description:Binding affinity for human 5-hydroxytryptamine 2A receptorMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2C(Homo sapiens (Human))
Merck Sharp And Dohme Research Laboratories
Curated by ChEMBL
Merck Sharp And Dohme Research Laboratories
Curated by ChEMBL
Affinity DataKi: 0.550nMAssay Description:Binding affinity towards human 5-hydroxytryptamine 2C receptorMore data for this Ligand-Target Pair
TargetD(2) dopamine receptor(Homo sapiens (Human))
Merck Sharp And Dohme Research Laboratories
Curated by ChEMBL
Merck Sharp And Dohme Research Laboratories
Curated by ChEMBL
Affinity DataKi: 2.80nMAssay Description:Binding affinity towards human D2 dopamine receptor.More data for this Ligand-Target Pair
TargetD(1A) dopamine receptor(Homo sapiens (Human))
Merck Sharp And Dohme Research Laboratories
Curated by ChEMBL
Merck Sharp And Dohme Research Laboratories
Curated by ChEMBL
Affinity DataKi: 9.5nMAssay Description:Binding affinity against dopamine receptor D1More data for this Ligand-Target Pair
TargetD(3) dopamine receptor(Homo sapiens (Human))
Merck Sharp And Dohme Research Laboratories
Curated by ChEMBL
Merck Sharp And Dohme Research Laboratories
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Binding affinity towards human dopamine receptor D3More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1A(Homo sapiens (Human))
Merck Sharp And Dohme Research Laboratories
Curated by ChEMBL
Merck Sharp And Dohme Research Laboratories
Curated by ChEMBL
Affinity DataKi: 37nMAssay Description:Binding affinity towards human 5-hydroxytryptamine 1 receptorMore data for this Ligand-Target Pair
TargetD(4) dopamine receptor(Homo sapiens (Human))
Merck Sharp And Dohme Research Laboratories
Curated by ChEMBL
Merck Sharp And Dohme Research Laboratories
Curated by ChEMBL
Affinity DataKi: 39nMAssay Description:Binding affinity towards human dopamine receptor D4More data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
Merck Sharp And Dohme Research Laboratories
Curated by ChEMBL
Merck Sharp And Dohme Research Laboratories
Curated by ChEMBL
Affinity DataKi: 510nMAssay Description:Binding affinity towards human H1 receptorMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M1(Homo sapiens (Human))
Merck Sharp And Dohme Research Laboratories
Curated by ChEMBL
Merck Sharp And Dohme Research Laboratories
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Binding affinity towards human M1 receptor.More data for this Ligand-Target Pair