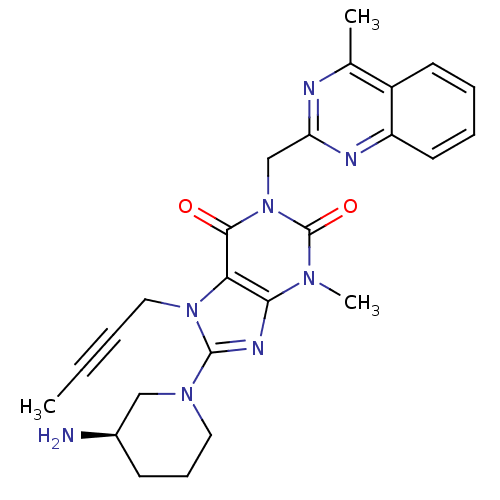
BDBM50228403 (R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-methyl-1-((4-methylquinazolin-2-yl)methyl)-1H-purine-2,6(3H,7H)-dione::8-[(3R)-3-Aminopiperidin-1-yl]-7-but-2-yn-1-yl-3-methyl-1-[(4-methylquinazolin-2-yl)methyl]-3,7-dihydro-1H-purine-2,6-dione::CHEMBL237500::LINAGLIPTIN::US10202383, Example 2(142)::US10358449, Linagliptin::US9255098, Linagliptin::US9321791, 2(142)::US9556175, 2(142)
SMILES CC#CCn1c(nc2n(C)c(=O)n(Cc3nc(C)c4ccccc4n3)c(=O)c12)N1CCC[C@@H](N)C1
InChI Key InChIKey=LTXREWYXXSTFRX-QGZVFWFLSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50228403
Found 3 hits for monomerid = 50228403
Affinity DataIC50: 295nMAssay Description:Inhibition of M1 receptorMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Argenta Discovery
Curated by ChEMBL
Argenta Discovery
Curated by ChEMBL
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of human ERG by dofetilide binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus systemMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)