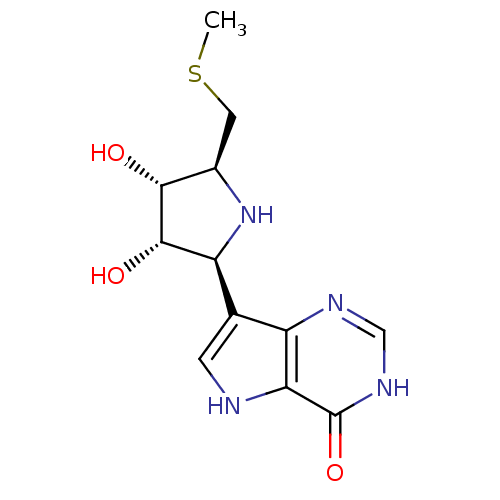
BDBM50247149 5'-Methylthio-ImmH::CHEMBL473929::US9290501, (A)
SMILES CSC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1c[nH]c2c1nc[nH]c2=O
InChI Key InChIKey=CEGIKIXYDFDYDN-RXDXJJGDSA-N
Data 4 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50247149
Found 4 hits for monomerid = 50247149
TargetS-methyl-5'-thioinosine phosphorylase(Pseudomonas aeruginosa)
Albert Einstein College of Medicine
US Patent
Albert Einstein College of Medicine
US Patent
Affinity DataKi: 0.0760nM ΔG°: -13.8kcal/molepH: 7.4 T: 2°CAssay Description:Assays for slow-onset inhibitors were carried out by adding 1 nM PaMTIP into reaction mixtures at 25 °C. containing 100 mM Hepes, pH 7.4, 100 mM ...More data for this Ligand-Target Pair
Affinity DataKi: 2.70nMAssay Description:Inhibition of Plasmodium falciparum 3D7 PNP expressed in Escherichia coli assessed as reduction in uric acid formation by spectrophotometric methodMore data for this Ligand-Target Pair
TargetPurine nucleoside phosphorylase(Homo sapiens (Human))
Albert Einstein College Of Medicine Of Yeshiva University
Curated by ChEMBL
Albert Einstein College Of Medicine Of Yeshiva University
Curated by ChEMBL
Affinity DataKi: 101nMAssay Description:Initial binding affinity to wild type human PNPMore data for this Ligand-Target Pair
TargetPurine nucleoside phosphorylase(Homo sapiens (Human))
Albert Einstein College Of Medicine Of Yeshiva University
Curated by ChEMBL
Albert Einstein College Of Medicine Of Yeshiva University
Curated by ChEMBL
Affinity DataKi: 303nMAssay Description:Inhibition of human PNP expressed in Escherichia coli assessed as reduction in uric acid formation by spectrophotometric methodMore data for this Ligand-Target Pair