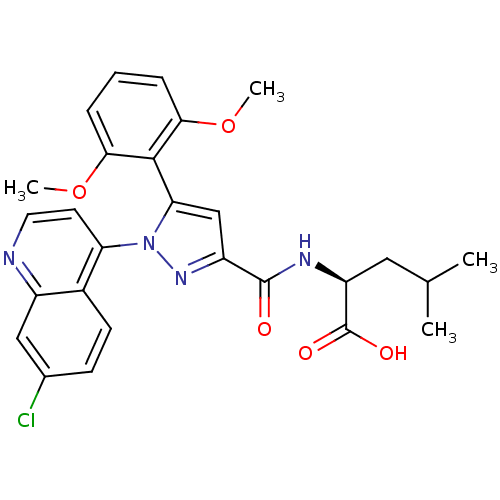
BDBM50248035 (2S)-2-(1-(7-chloroquinolin-4-yl)-5-(2,6-dimethoxyphenyl)-1H-pyrazole-3-carboxamido)-4-methylpentanoic acid::CHEMBL508044
SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccnc2cc(Cl)ccc12)C(=O)N[C@@H](CC(C)C)C(O)=O
InChI Key InChIKey=ZQUSYVORYNBGLG-FQEVSTJZSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 12 hits for monomerid = 50248035
Found 12 hits for monomerid = 50248035
TargetNeurotensin receptor type 1(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataKi: 3.30E+3nMAssay Description:Inhibition of NTS1 receptor (unknown origin)More data for this Ligand-Target Pair
TargetMu-type opioid receptor(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataKi: 3.40E+3nMAssay Description:Inhibition of MOR (unknown origin)More data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataKi: 5.20E+3nMAssay Description:Inhibition of DOR (unknown origin)More data for this Ligand-Target Pair
TargetSodium-dependent dopamine transporter(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Inhibition of DAT (unknown origin)More data for this Ligand-Target Pair
TargetNeurotensin receptor type 1(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataEC50: 67nMAssay Description:Agonist activity at NTR1 (unknown origin) expressed in CHOK1 cells by calcium mobilization assayMore data for this Ligand-Target Pair
TargetNeurotensin receptor type 1(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataEC50: 750nMAssay Description:Agonist activity at NTR1 (unknown origin) expressed in human U2OS cells coexpressing beta-arrestin by GFP reporter gene assayMore data for this Ligand-Target Pair
TargetNeurotensin receptor type 1(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataEC50: >3.30E+4nMAssay Description:Agonist activity at NTR1 (unknown origin) expressed in CHOK1 cells by beta-arrestin assayMore data for this Ligand-Target Pair
TargetNeurotensin receptor type 2(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataEC50: >8.00E+4nMAssay Description:Agonist activity at NTR2 in human U2OS cells after 1 hr by beta-arrestin GFP reporter gene assayMore data for this Ligand-Target Pair
TargetG-protein coupled receptor 35(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataEC50: >4.00E+4nMAssay Description:Agonist activity at GPR35 in human U2OS cells after 1 hr by beta-arrestin GFP reporter gene assayMore data for this Ligand-Target Pair
TargetNeurotensin receptor type 1(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataEC50: 750nMAssay Description:Agonist activity at NTR1 in human U2OS cells after 1 hr by beta-arrestin GFP reporter gene assayMore data for this Ligand-Target Pair
TargetNeurotensin receptor type 1(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataEC50: >3.30E+4nMAssay Description:Agonist activity at NTR1 (unknown origin) expressed in CHO-K1 cells coexpressing beta-arrestin/N-terminal deletion mutant of beta-galactosidase fusio...More data for this Ligand-Target Pair
TargetNeurotensin receptor type 1(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataEC50: <156nMAssay Description:Agonist activity at NTR1 (unknown origin) expressed in CHO cells assessed as Ca2+ mobilization by Fluo-4 NW dye-based fluorescence assayMore data for this Ligand-Target Pair