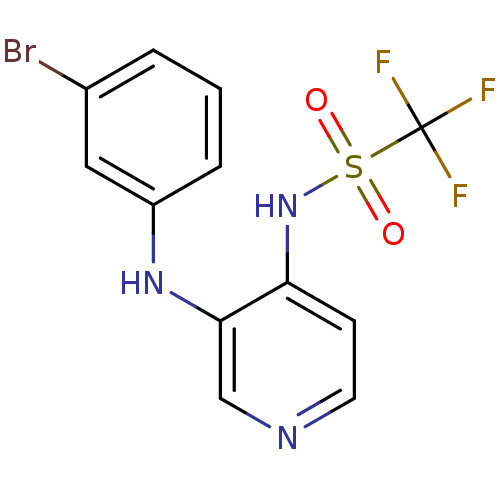
BDBM50311392 CHEMBL1079335::N-[3-(3-Bromophenylamino)-4-pyridinyl]trifluoromethanesulfonamide
SMILES FC(F)(F)S(=O)(=O)Nc1ccncc1Nc1cccc(Br)c1
InChI Key InChIKey=WYMPMNZWERFHGE-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50311392
Found 4 hits for monomerid = 50311392
Affinity DataIC50: 120nMAssay Description:Inhibition of COX2 in LPS-stimulated human whole blood assessed as PGE2 production by enzyme immunoassayMore data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research And Development
Curated by ChEMBL
TargetProstaglandin G/H synthase 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 910nMAssay Description:Inhibition of COX1 in human whole blood assessed as TXB2 production by enzyme immunoassayMore data for this Ligand-Target Pair