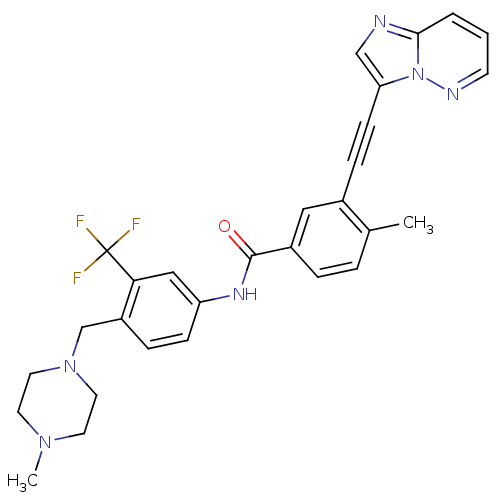
BDBM50322535 3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N-{4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl}benzamide::3-[2-(Imidazo[1,2-b]pyridazin-3-yl)ethynyl]-4-methyl-N-{4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl}benzamide::CHEMBL1171837::PONATINIB::US10464902, Ponatinib::US9255107, AP24534
SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1
InChI Key InChIKey=PHXJVRSECIGDHY-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50322535
Found 3 hits for monomerid = 50322535
Affinity DataKd: 0.700nMAssay Description:Inhibitor selectivity profiles were obtained through Luceome Biotechnologies (Tuscon, AZ). Each inhibitor was screened at 0.5 μM against a panel...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src [251-533](Gallus gallus (Chicken))
Stony Brook University
Stony Brook University
Affinity DataKd: 0.700nMAssay Description:Inhibitor selectivity profiles were obtained through Luceome Biotechnologies (Tuscon, AZ). Each inhibitor was screened at 0.5 μM against phospho...More data for this Ligand-Target Pair
Affinity DataKd: 6.90nMAssay Description:Kinobeads competition assays were performed in 96-well format as previously described using mixed protein lysates of four cancer cell lines (K-562, M...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)