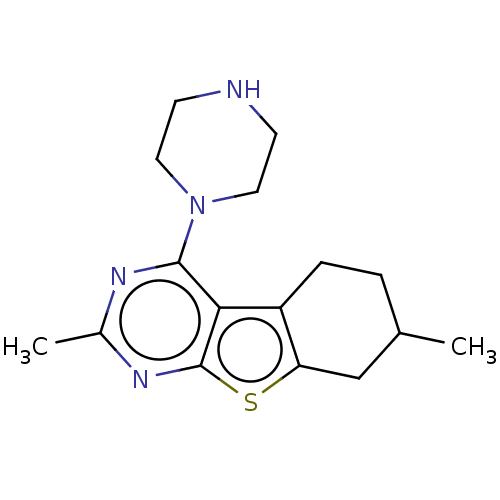
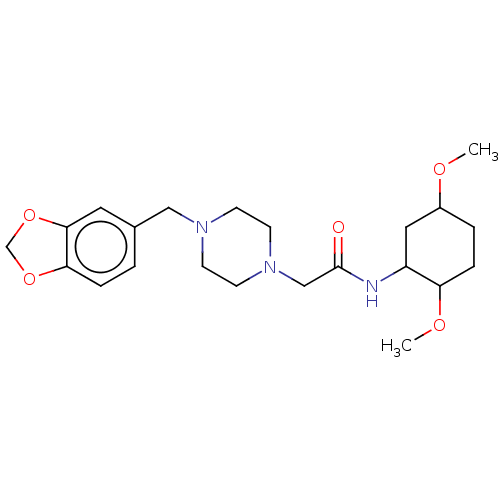
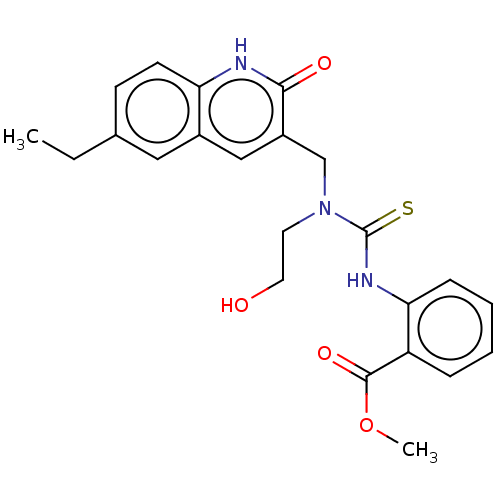
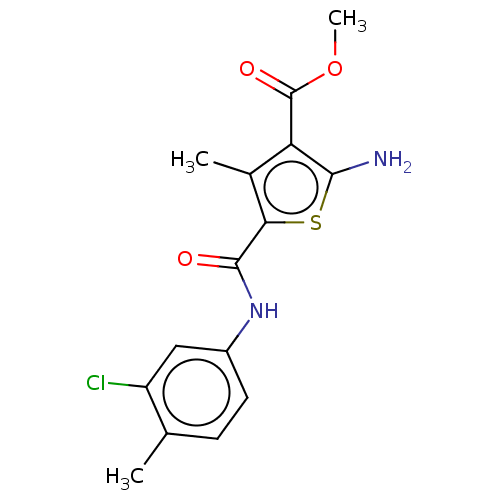
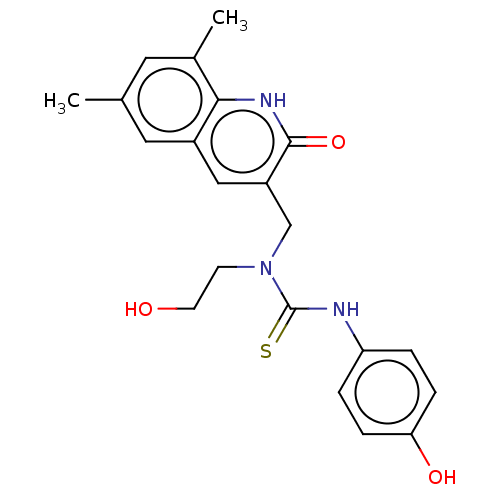
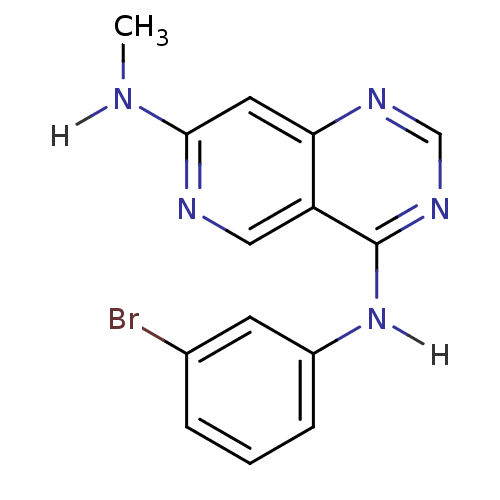
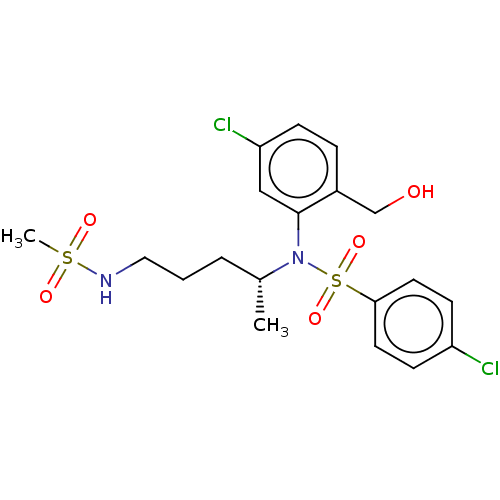
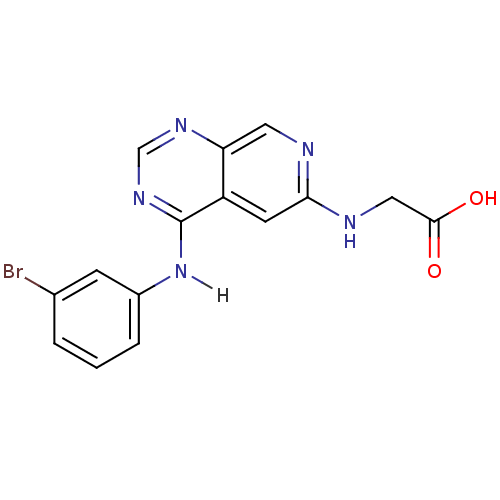
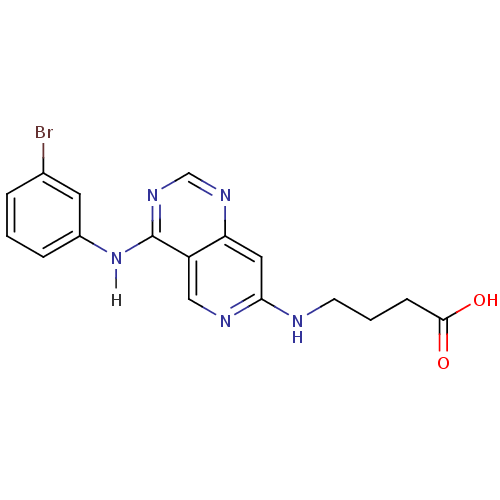
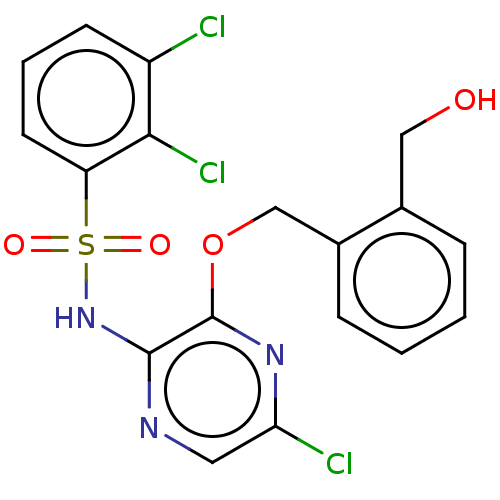
TargetBeta-glucuronidase(Escherichia coli (Enterobacteria))
University of North Carolina At Chapel Hill
University of North Carolina At Chapel Hill
Affinity DataKi: 160nMAssay Description:Reactions were conducted similarly to the kinetic assays but the reaction consisted of 10 無 assay buffer, 5 無 inhibitor solution, 5 無 of 10 nM enz...More data for this Ligand-Target Pair
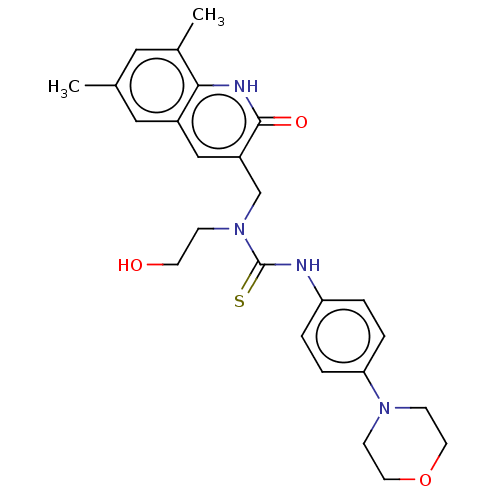
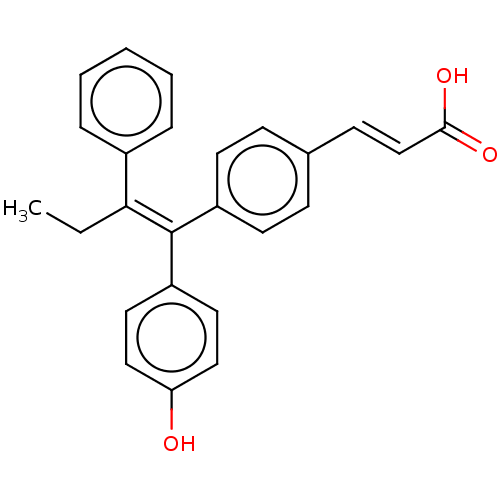
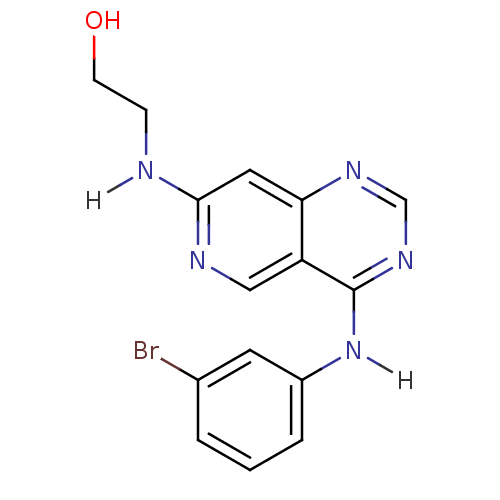
TargetBeta-glucuronidase(Escherichia coli (Enterobacteria))
University of North Carolina At Chapel Hill
University of North Carolina At Chapel Hill
Affinity DataKi: 210nMAssay Description:Reactions were conducted similarly to the kinetic assays but the reaction consisted of 10 無 assay buffer, 5 無 inhibitor solution, 5 無 of 10 nM enz...More data for this Ligand-Target Pair
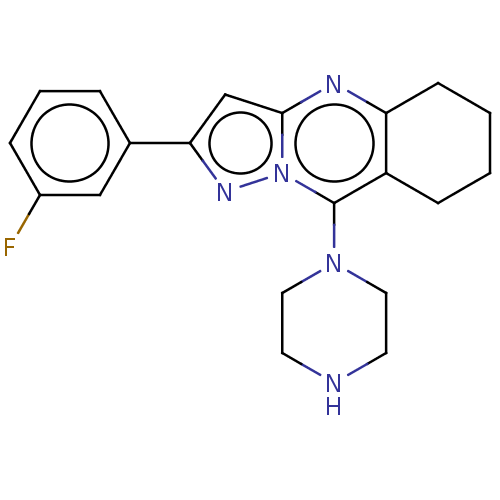
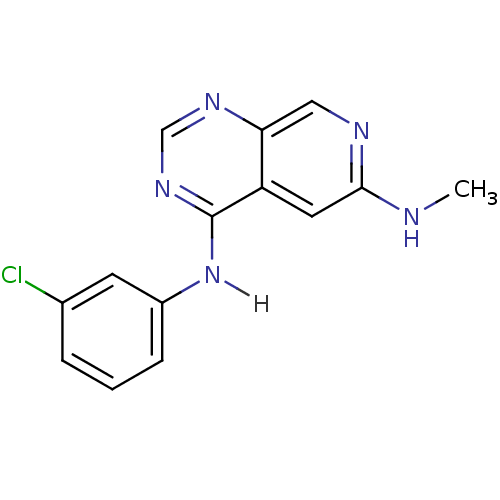
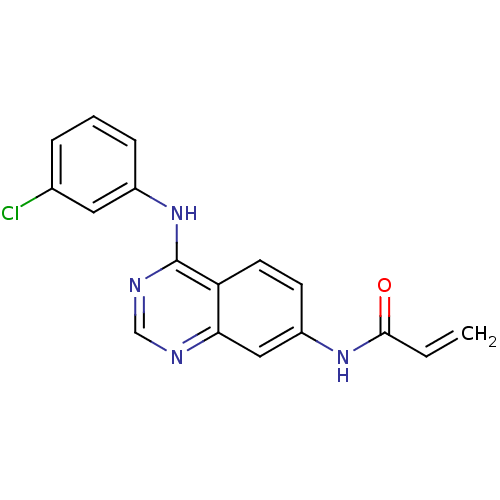
TargetBeta-glucuronidase(Escherichia coli (Enterobacteria))
University of North Carolina At Chapel Hill
University of North Carolina At Chapel Hill
Affinity DataKi: 220nMAssay Description:Reactions were conducted similarly to the kinetic assays but the reaction consisted of 10 無 assay buffer, 5 無 inhibitor solution, 5 無 of 10 nM enz...More data for this Ligand-Target Pair
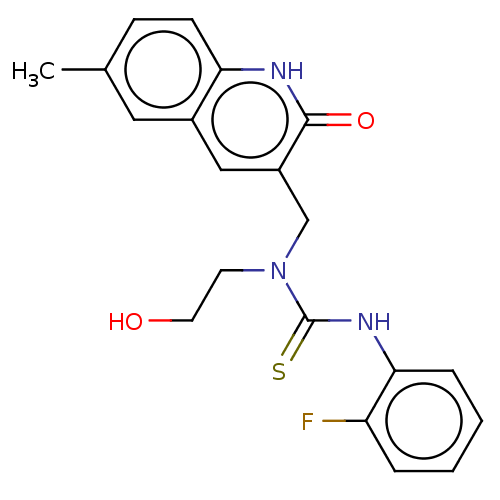
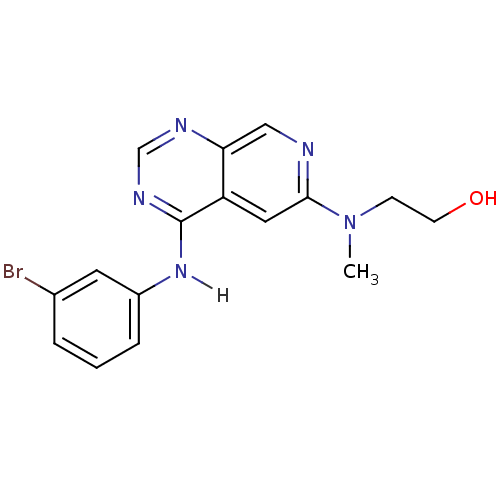
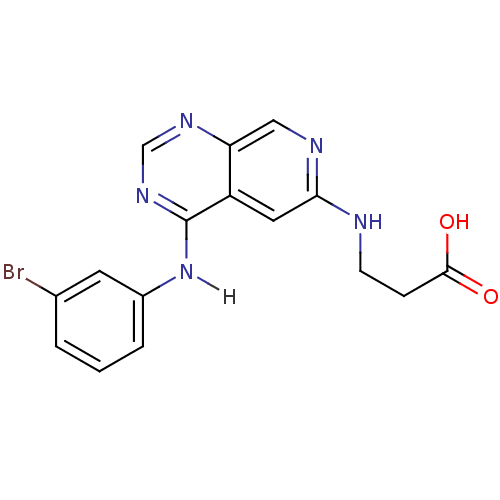
TargetBeta-galactosidase [V151I,I185V](Clostridium perfringens (Firmicutes))
University of North Carolina At Chapel Hill
University of North Carolina At Chapel Hill
Affinity DataKi: 540nMAssay Description:Reactions were conducted similarly to the kinetic assays but the reaction consisted of 10 無 assay buffer, 5 無 inhibitor solution, 5 無 of 10 nM enz...More data for this Ligand-Target Pair
TargetBeta-glucuronidase(Escherichia coli (Enterobacteria))
University of North Carolina At Chapel Hill
University of North Carolina At Chapel Hill
Affinity DataKi: 610nMAssay Description:Reactions were conducted similarly to the kinetic assays but the reaction consisted of 10 無 assay buffer, 5 無 inhibitor solution, 5 無 of 10 nM enz...More data for this Ligand-Target Pair
TargetBeta-glucuronidase(Escherichia coli (Enterobacteria))
University of North Carolina At Chapel Hill
University of North Carolina At Chapel Hill
Affinity DataKi: 670nMAssay Description:Reactions were conducted similarly to the kinetic assays but the reaction consisted of 10 無 assay buffer, 5 無 inhibitor solution, 5 無 of 10 nM enz...More data for this Ligand-Target Pair
TargetBeta-glucuronidase(Escherichia coli (Enterobacteria))
University of North Carolina At Chapel Hill
University of North Carolina At Chapel Hill
Affinity DataKi: 680nMAssay Description:Reactions were conducted similarly to the kinetic assays but the reaction consisted of 10 無 assay buffer, 5 無 inhibitor solution, 5 無 of 10 nM enz...More data for this Ligand-Target Pair
TargetBeta-galactosidase(Streptococcus agalactiae (Firmicutes))
University of North Carolina At Chapel Hill
University of North Carolina At Chapel Hill
Affinity DataKi: 810nMAssay Description:Reactions were conducted similarly to the kinetic assays but the reaction consisted of 10 無 assay buffer, 5 無 inhibitor solution, 5 無 of 10 nM enz...More data for this Ligand-Target Pair
TargetBeta-glucuronidase(Escherichia coli (Enterobacteria))
University of North Carolina At Chapel Hill
University of North Carolina At Chapel Hill
Affinity DataKi: 960nMAssay Description:Reactions were conducted similarly to the kinetic assays but the reaction consisted of 10 無 assay buffer, 5 無 inhibitor solution, 5 無 of 10 nM enz...More data for this Ligand-Target Pair
TargetBeta-galactosidase [V151I,I185V](Clostridium perfringens (Firmicutes))
University of North Carolina At Chapel Hill
University of North Carolina At Chapel Hill
Affinity DataKi: 970nMAssay Description:Reactions were conducted similarly to the kinetic assays but the reaction consisted of 10 無 assay buffer, 5 無 inhibitor solution, 5 無 of 10 nM enz...More data for this Ligand-Target Pair
TargetBeta-galactosidase [V151I,I185V](Clostridium perfringens (Firmicutes))
University of North Carolina At Chapel Hill
University of North Carolina At Chapel Hill
Affinity DataKi: 1.10E+3nMAssay Description:Reactions were conducted similarly to the kinetic assays but the reaction consisted of 10 無 assay buffer, 5 無 inhibitor solution, 5 無 of 10 nM enz...More data for this Ligand-Target Pair
TargetBeta-galactosidase(Streptococcus agalactiae (Firmicutes))
University of North Carolina At Chapel Hill
University of North Carolina At Chapel Hill
Affinity DataKi: 1.40E+3nMAssay Description:Reactions were conducted similarly to the kinetic assays but the reaction consisted of 10 無 assay buffer, 5 無 inhibitor solution, 5 無 of 10 nM enz...More data for this Ligand-Target Pair
TargetBeta-glucuronidase(Escherichia coli (Enterobacteria))
University of North Carolina At Chapel Hill
University of North Carolina At Chapel Hill
Affinity DataKi: 1.40E+3nMAssay Description:Reactions were conducted similarly to the kinetic assays but the reaction consisted of 10 無 assay buffer, 5 無 inhibitor solution, 5 無 of 10 nM enz...More data for this Ligand-Target Pair
TargetBeta-glucuronidase(Escherichia coli (Enterobacteria))
University of North Carolina At Chapel Hill
University of North Carolina At Chapel Hill
Affinity DataKi: 1.90E+3nMAssay Description:Reactions were conducted similarly to the kinetic assays but the reaction consisted of 10 無 assay buffer, 5 無 inhibitor solution, 5 無 of 10 nM enz...More data for this Ligand-Target Pair
TargetBeta-glucuronidase(Escherichia coli (Enterobacteria))
University of North Carolina At Chapel Hill
University of North Carolina At Chapel Hill
Affinity DataKi: 1.90E+3nMAssay Description:Reactions were conducted similarly to the kinetic assays but the reaction consisted of 10 無 assay buffer, 5 無 inhibitor solution, 5 無 of 10 nM enz...More data for this Ligand-Target Pair
TargetBeta-galactosidase(Streptococcus agalactiae (Firmicutes))
University of North Carolina At Chapel Hill
University of North Carolina At Chapel Hill
Affinity DataKi: 2.80E+3nMAssay Description:Reactions were conducted similarly to the kinetic assays but the reaction consisted of 10 無 assay buffer, 5 無 inhibitor solution, 5 無 of 10 nM enz...More data for this Ligand-Target Pair
TargetBeta-galactosidase(Streptococcus agalactiae (Firmicutes))
University of North Carolina At Chapel Hill
University of North Carolina At Chapel Hill
Affinity DataKi: 3.00E+3nMAssay Description:Reactions were conducted similarly to the kinetic assays but the reaction consisted of 10 無 assay buffer, 5 無 inhibitor solution, 5 無 of 10 nM enz...More data for this Ligand-Target Pair
TargetBeta-galactosidase [V151I,I185V](Clostridium perfringens (Firmicutes))
University of North Carolina At Chapel Hill
University of North Carolina At Chapel Hill
Affinity DataKi: 6.10E+3nMAssay Description:Reactions were conducted similarly to the kinetic assays but the reaction consisted of 10 無 assay buffer, 5 無 inhibitor solution, 5 無 of 10 nM enz...More data for this Ligand-Target Pair
TargetBeta-galactosidase [V151I,I185V](Clostridium perfringens (Firmicutes))
University of North Carolina At Chapel Hill
University of North Carolina At Chapel Hill
Affinity DataKi: 7.80E+3nMAssay Description:Reactions were conducted similarly to the kinetic assays but the reaction consisted of 10 無 assay buffer, 5 無 inhibitor solution, 5 無 of 10 nM enz...More data for this Ligand-Target Pair
TargetBeta-galactosidase(Streptococcus agalactiae (Firmicutes))
University of North Carolina At Chapel Hill
University of North Carolina At Chapel Hill
Affinity DataKi: 1.10E+4nMAssay Description:Reactions were conducted similarly to the kinetic assays but the reaction consisted of 10 無 assay buffer, 5 無 inhibitor solution, 5 無 of 10 nM enz...More data for this Ligand-Target Pair
TargetBeta-galactosidase [V151I,I185V](Clostridium perfringens (Firmicutes))
University of North Carolina At Chapel Hill
University of North Carolina At Chapel Hill
Affinity DataKi: 2.40E+4nMAssay Description:Reactions were conducted similarly to the kinetic assays but the reaction consisted of 10 無 assay buffer, 5 無 inhibitor solution, 5 無 of 10 nM enz...More data for this Ligand-Target Pair
TargetBeta-galactosidase(Streptococcus agalactiae (Firmicutes))
University of North Carolina At Chapel Hill
University of North Carolina At Chapel Hill
Affinity DataKi: 3.60E+4nMAssay Description:Reactions were conducted similarly to the kinetic assays but the reaction consisted of 10 無 assay buffer, 5 無 inhibitor solution, 5 無 of 10 nM enz...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00600nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00800nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0900nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 0.130nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 0.130nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 0.150nMAssay Description:Binding affinity to ER alpha (unknown origin) by LanthaScreen TR-FRET competitive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.170nMpH: 7.4 T: 2°CAssay Description:Enzyme assays for IC50 determinations were performed in 96-well filter plates. IC50 is the inhibitor concentration which inhibits 50% of kinase activ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.180nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 0.190nMAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 0.190nMAssay Description:Inhibition of human gamma-secretase expressed in IMR32 cell membranes using MBPC-125 Swedish as substrate assessed as inhibition of amyloid beta40 pr...More data for this Ligand-Target Pair
Affinity DataIC50: 0.190nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Inhibition of erbB1 fusion protein expressed in baculovirus by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.220nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 0.230nMAssay Description:Inhibition of human gamma secretase in H4 cells assessed as reduction of amyloid beta-40 levelsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.240nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 0.25nMAssay Description:Downregulation of ERalpha in human MCF7 cells incubated for 18 to 22 hrs by immunofluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.25nMAssay Description:Inhibition of phosphorylation of glutamic acid/tyrosine random copolymer by isolated epidermal growth factor receptor (EGFR)More data for this Ligand-Target Pair
Affinity DataIC50: 0.270nMAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 0.280nMAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 0.280nMAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Inhibition of erbB1 fusion protein expressed in baculovirus by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 0.316nMAssay Description:Antagonist activity at recombinant human CCR4 expressed in CHO-K1 cells assessed as inhibition of CCL22 induced Ca2+ mobilization after 2 hrs by FMAT...More data for this Ligand-Target Pair
Affinity DataIC50: 0.320nMAssay Description:Inhibition of human gamma secretase in H4 cells assessed as reduction of amyloid beta-40 levelsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.350nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)