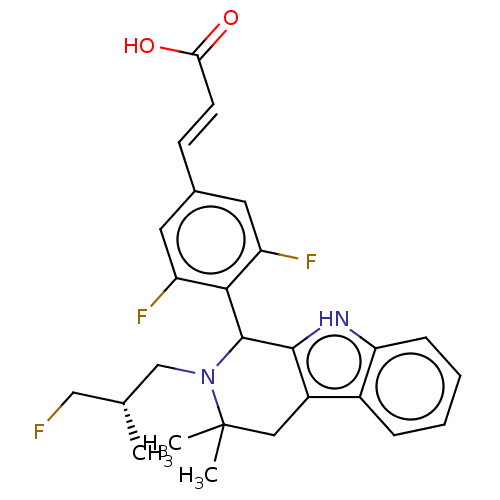
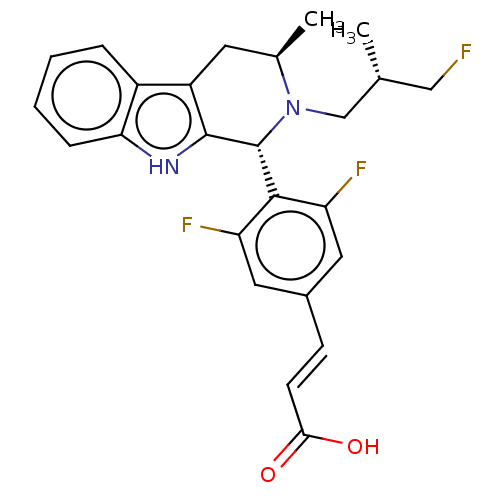
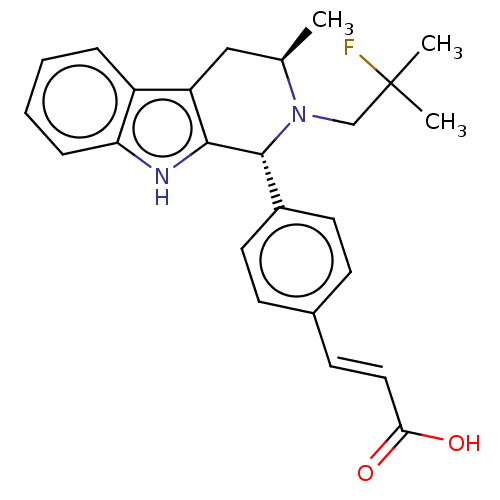
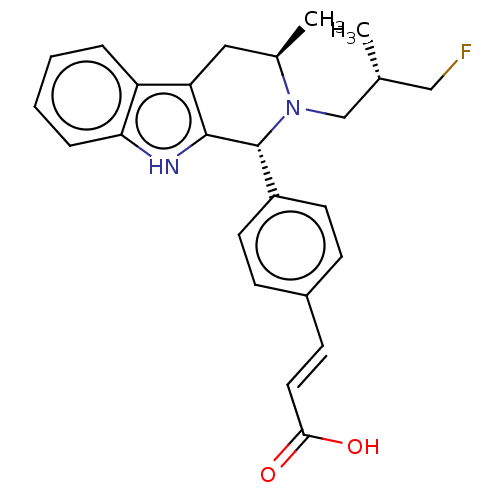
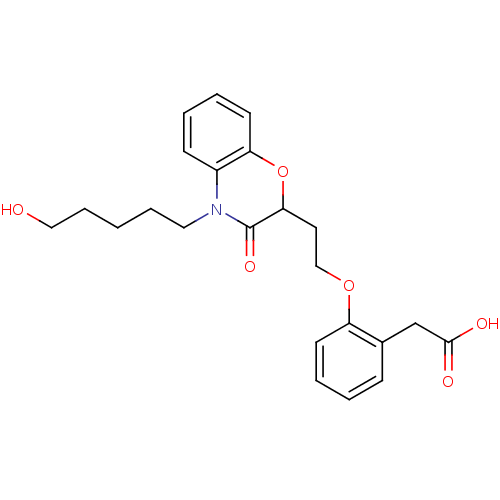
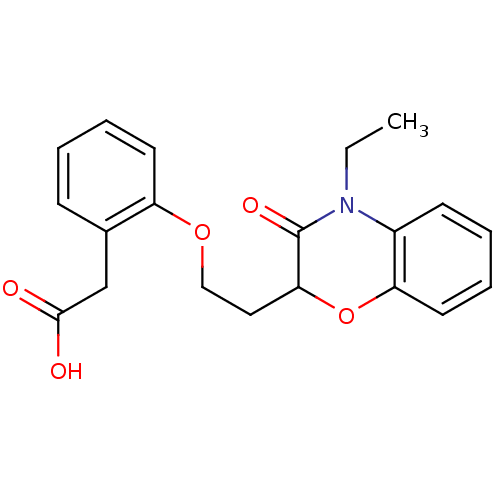
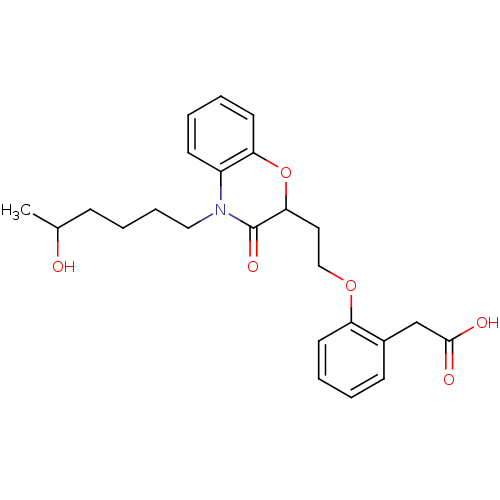
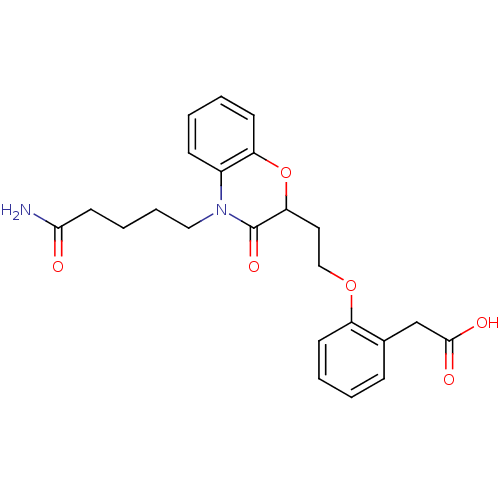
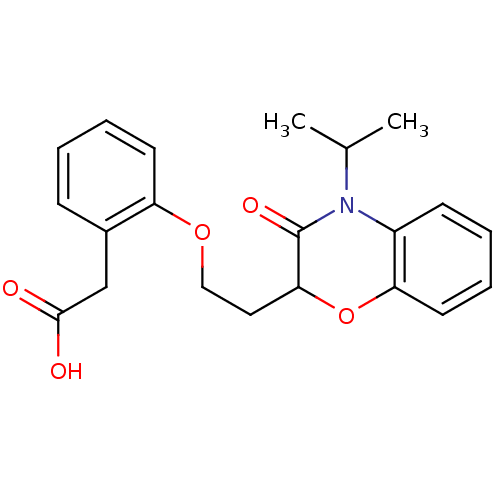
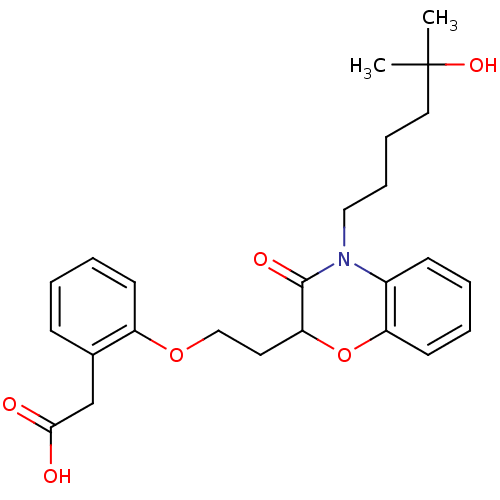
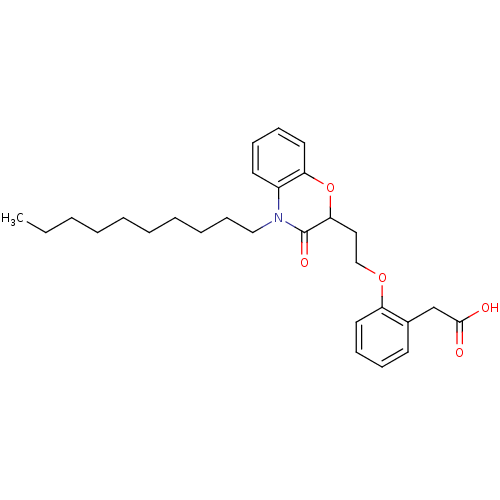
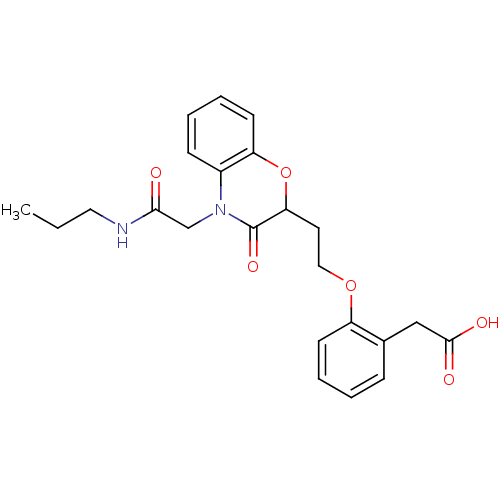
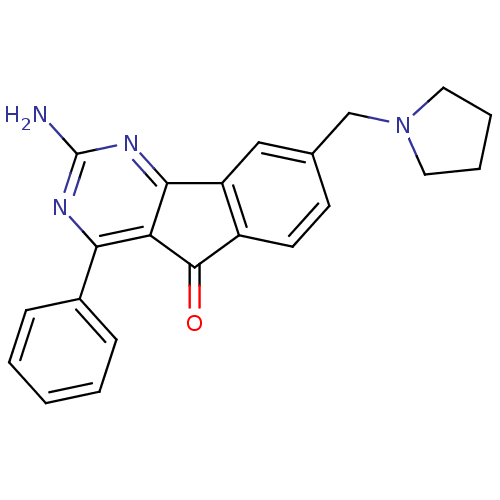
TargetAdenosine receptor A2a(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 4.10nMAssay Description:Antagonist activity at adenosine A2A receptorChecked by AuthorMore data for this Ligand-Target Pair
TargetAdenosine receptor A1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 17nMAssay Description:Antagonist activity at adenosine A1 receptorChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataIC50: 140nMAssay Description:The ability of compounds to down-regulate Estrogen Receptor (ER) numbers was assessed in a cell based immuno-fluorescence assay using the MCF-7 human...More data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:The ability of compounds to bind to isolated Estrogen Receptor Alpha Ligand binding domain (ER alpha LBD (GST)) was assessed in competition assays us...More data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:The ability of compounds to down-regulate Estrogen Receptor (ER) numbers was assessed in a cell based immuno-fluorescence assay using the MCF-7 human...More data for this Ligand-Target Pair
Affinity DataIC50: 440nMAssay Description:The ability of compounds to down-regulate Estrogen Receptor (ER) numbers was assessed in a cell based immuno-fluorescence assay using the MCF-7 human...More data for this Ligand-Target Pair
Affinity DataIC50: 570nMAssay Description:The ability of compounds to down-regulate Estrogen Receptor (ER) numbers was assessed in a cell based immuno-fluorescence assay using the MCF-7 human...More data for this Ligand-Target Pair
Affinity DataIC50: <640nMAssay Description:The ability of compounds to bind to isolated Estrogen Receptor Alpha Ligand binding domain (ER alpha LBD (GST)) was assessed in competition assays us...More data for this Ligand-Target Pair
Affinity DataIC50: 850nMAssay Description:The ability of compounds to down-regulate Estrogen Receptor (ER) numbers was assessed in a cell based immuno-fluorescence assay using the MCF-7 human...More data for this Ligand-Target Pair
Affinity DataIC50: 990nMAssay Description:The ability of compounds to down-regulate Estrogen Receptor (ER) numbers was assessed in a cell based immuno-fluorescence assay using the MCF-7 human...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:The ability of compounds to bind to isolated Estrogen Receptor Alpha Ligand binding domain (ER alpha LBD (GST)) was assessed in competition assays us...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:The ability of compounds to bind to isolated Estrogen Receptor Alpha Ligand binding domain (ER alpha LBD (GST)) was assessed in competition assays us...More data for this Ligand-Target Pair
Affinity DataIC50: <1.20E+3nMAssay Description:The ability of compounds to bind to isolated Estrogen Receptor Alpha Ligand binding domain (ER alpha LBD (GST)) was assessed in competition assays us...More data for this Ligand-Target Pair
Affinity DataIC50: <1.30E+3nMAssay Description:The ability of compounds to bind to isolated Estrogen Receptor Alpha Ligand binding domain (ER alpha LBD (GST)) was assessed in competition assays us...More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:The ability of compounds to down-regulate Estrogen Receptor (ER) numbers was assessed in a cell based immuno-fluorescence assay using the MCF-7 human...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMAssay Description:The ability of compounds to bind to isolated Estrogen Receptor Alpha Ligand binding domain (ER alpha LBD (GST)) was assessed in competition assays us...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:The ability of compounds to down-regulate Estrogen Receptor (ER) numbers was assessed in a cell based immuno-fluorescence assay using the MCF-7 human...More data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+3nMAssay Description:The ability of compounds to bind to isolated Estrogen Receptor Alpha Ligand binding domain (ER alpha LBD (GST)) was assessed in competition assays us...More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:The ability of compounds to down-regulate Estrogen Receptor (ER) numbers was assessed in a cell based immuno-fluorescence assay using the MCF-7 human...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:The ability of compounds to bind to isolated Estrogen Receptor Alpha Ligand binding domain (ER alpha LBD (GST)) was assessed in competition assays us...More data for this Ligand-Target Pair
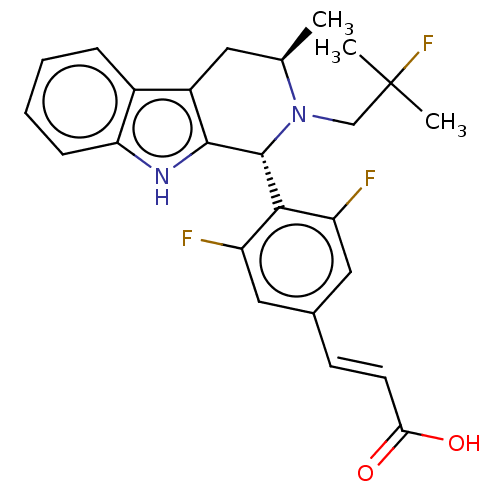
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: 10nMAssay Description:Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesisMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: 274nMAssay Description:Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesisMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: 1.00E+3nMAssay Description:Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesisMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: >5.00E+3nMAssay Description:Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesisMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: 570nMAssay Description:Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesisMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: 718nMAssay Description:Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesisMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: 2.70E+3nMAssay Description:Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesisMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: >5.00E+3nMAssay Description:Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesisMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: >5.00E+3nMAssay Description:Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesisMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: 300nMAssay Description:Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesisMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: 179nMAssay Description:Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesisMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: 274nMAssay Description:Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesisMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: >5.00E+3nMAssay Description:Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesisMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: 1.00E+3nMAssay Description:Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesisMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: 1.00E+3nMAssay Description:Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesisMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: 152nMAssay Description:Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesisMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: 234nMAssay Description:Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesisMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: 295nMAssay Description:Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesisMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: >5.00E+3nMAssay Description:Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesisMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: >5.00E+3nMAssay Description:Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesisMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: 117nMAssay Description:Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesisMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: >5.00E+3nMAssay Description:Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesisMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: 1.00E+3nMAssay Description:Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesisMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: 208nMAssay Description:Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesisMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: 644nMAssay Description:Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesisMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: 260nMAssay Description:Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesisMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: 1.00E+3nMAssay Description:Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesisMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: >5.00E+3nMAssay Description:Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesisMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: >5.00E+3nMAssay Description:Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesisMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataEC50: >5.00E+3nMAssay Description:Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesisMore data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)