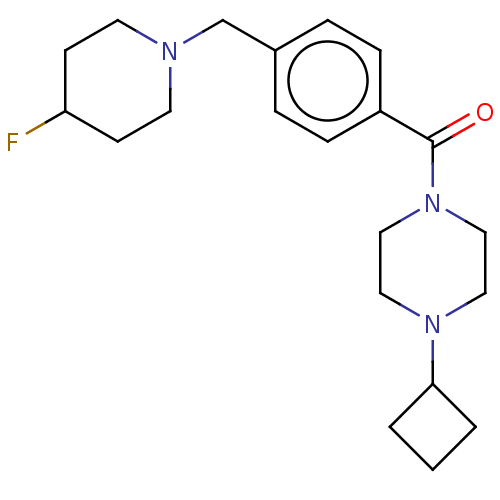
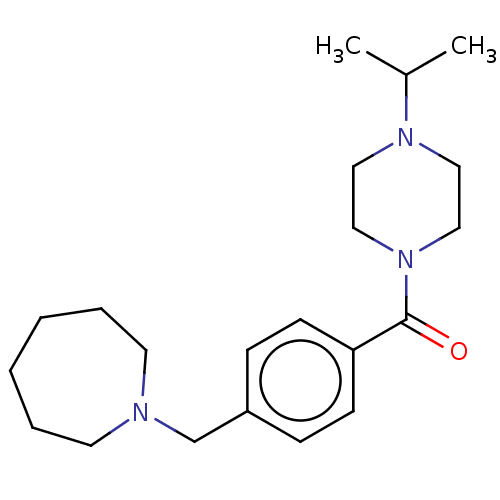
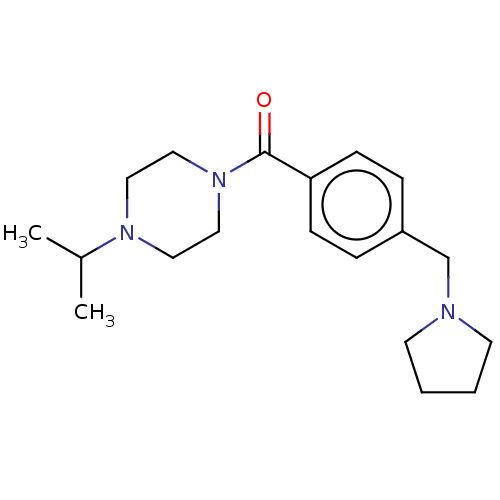
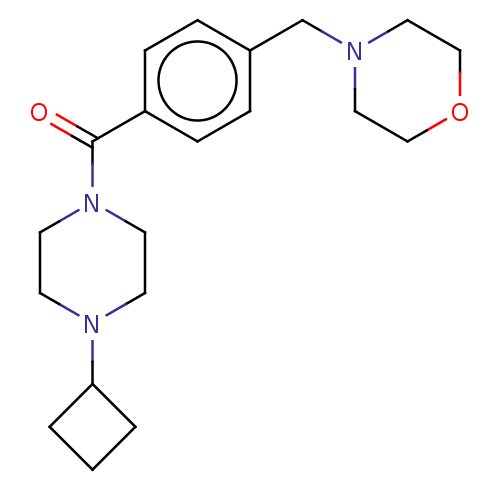
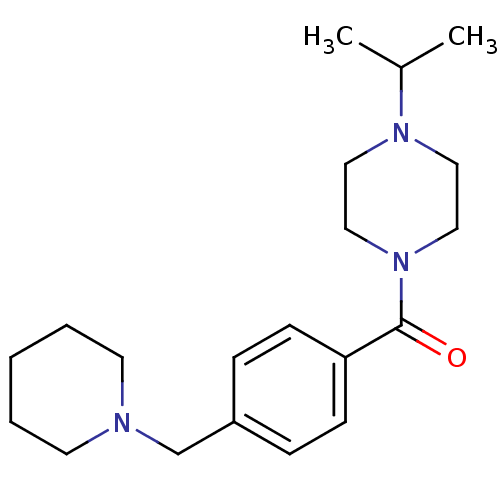
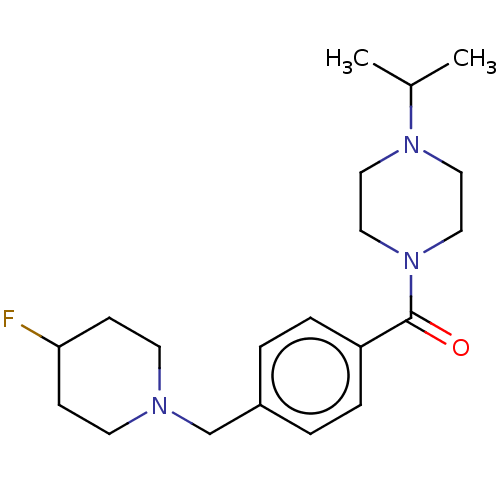
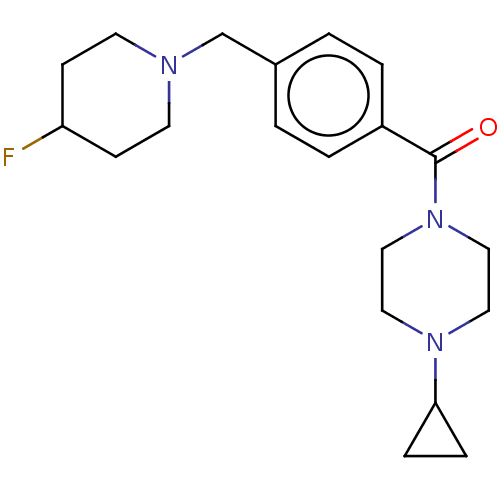
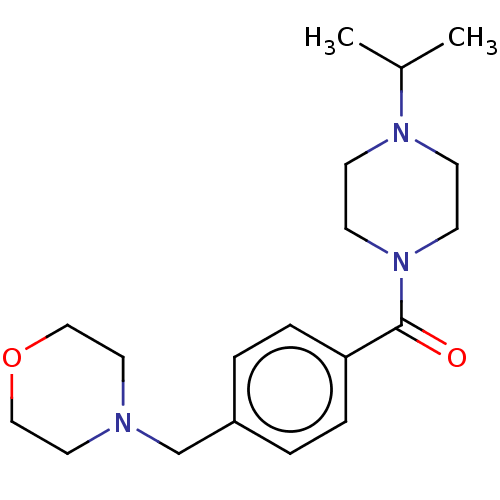
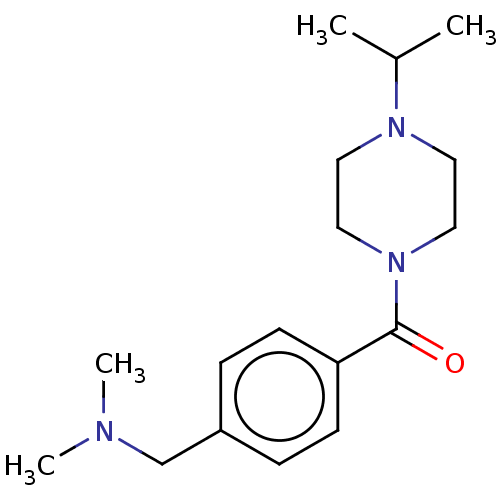
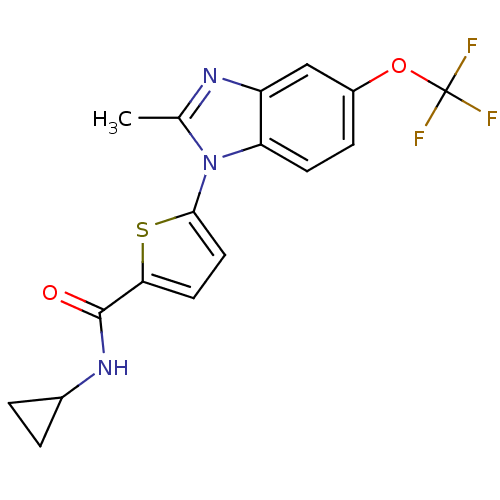
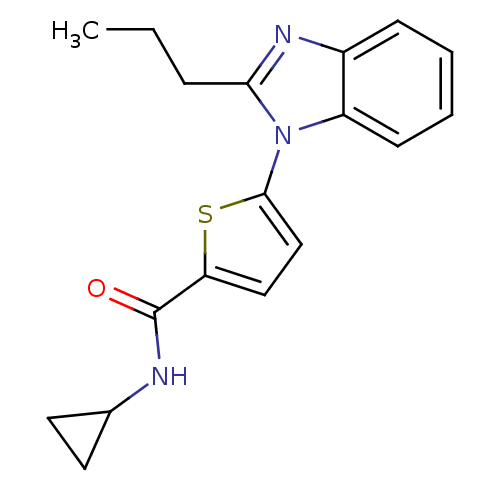
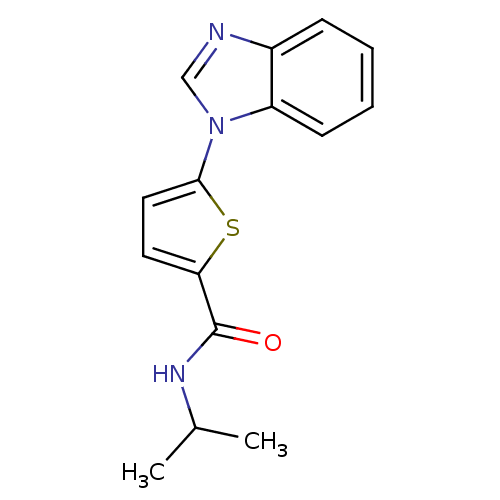
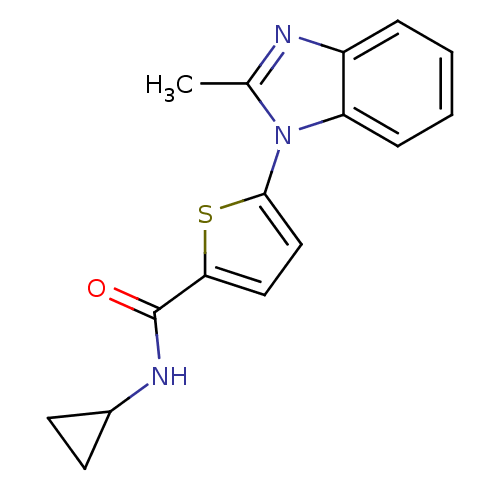
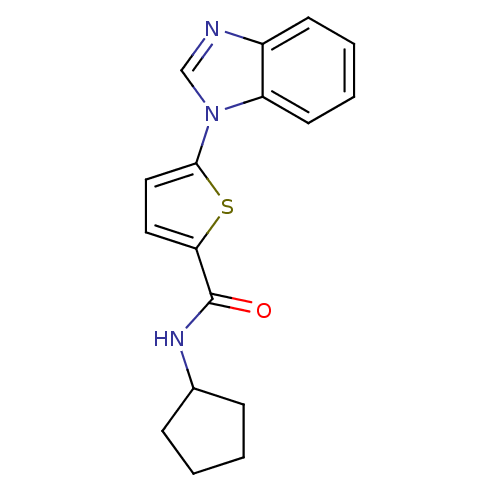
TargetHistamine H3 receptor(Homo sapiens (Human))
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 minsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 minsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 minsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Affinity DataKi: 1.30nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 minsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Affinity DataKi: 1.30nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 minsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Affinity DataKi: 1.5nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 minsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 minsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Affinity DataKi: 2.5nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 minsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Affinity DataKi: 5nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 minsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Affinity DataKi: 5.40nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 minsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Affinity DataKi: 6nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 minsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Affinity DataKi: 22nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 minsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Affinity DataKi: 24nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of human CYP2D6 using bufuralol as substrate after 10 mins by LC-MS/MS analysisMore data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 3.10E+3nMAssay Description:Inhibition of human DHODH after 20 mins by 2,6-dichloroindophenol-reduction based assayMore data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 5.60E+3nMAssay Description:Inhibition of human DHODH after 20 mins by 2,6-dichloroindophenol-reduction based assayMore data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 7.30E+3nMAssay Description:Inhibition of human DHODH after 20 mins by 2,6-dichloroindophenol-reduction based assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Displacement of [3H]-astemizole from human ERG expressed in HEK293 cells after 60 mins by scintillation counting analysisMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Displacement of [3H]-astemizole from human ERG expressed in HEK293 cells after 60 mins by scintillation counting analysisMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Displacement of [3H]-astemizole from human ERG expressed in HEK293 cells after 60 mins by scintillation counting analysisMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Displacement of [3H]-astemizole from human ERG expressed in HEK293 cells after 60 mins by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human CYP1A2 using phenacetin as substrate after 10 mins by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human CYP2D6 using bufuralol as substrate after 10 mins by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human CYP2D6 using bufuralol as substrate after 10 mins by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human CYP3A4 using midazolam as substrate after 5 mins by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human CYP3A4 using midazolam as substrate after 5 mins by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human CYP2C19 using S-mephenytoin as substrate after 45 mins by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human CYP2C19 using S-mephenytoin as substrate after 45 mins by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human CYP2C9 using diclofenac as substrate after 10 mins by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human CYP2C9 using diclofenac as substrate after 10 mins by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human CYP1A2 using phenacetin as substrate after 10 mins by LC-MS/MS analysisMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Displacement of [3H]-astemizole from human ERG expressed in HEK293 cells after 60 mins by scintillation counting analysisMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Displacement of [3H]-astemizole from human ERG expressed in HEK293 cells after 60 mins by scintillation counting analysisMore data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 1.49E+4nMAssay Description:Inhibition of human DHODH after 20 mins by 2,6-dichloroindophenol-reduction based assayMore data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 1.51E+4nMAssay Description:Inhibition of human DHODH after 20 mins by 2,6-dichloroindophenol-reduction based assayMore data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 1.72E+4nMAssay Description:Inhibition of human DHODH after 20 mins by 2,6-dichloroindophenol-reduction based assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Affinity DataIC50: 2.88E+4nMAssay Description:Inhibition of human ERG expressed in CHOK1 cells by whole cell voltage clamp assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Janssen Pharmaceutical Companies Of Johnson & Johnson
Curated by ChEMBL
Affinity DataIC50: >3.00E+4nMAssay Description:Displacement of [3H]-astemizole from human ERG expressed in HEK293 cells after 60 mins by scintillation counting analysisMore data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of human DHODH after 20 mins by 2,6-dichloroindophenol-reduction based assayMore data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of human DHODH after 20 mins by 2,6-dichloroindophenol-reduction based assayMore data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of human DHODH after 20 mins by 2,6-dichloroindophenol-reduction based assayMore data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of human DHODH after 20 mins by 2,6-dichloroindophenol-reduction based assayMore data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of human DHODH after 20 mins by 2,6-dichloroindophenol-reduction based assayMore data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of human DHODH after 20 mins by 2,6-dichloroindophenol-reduction based assayMore data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of human DHODH after 20 mins by 2,6-dichloroindophenol-reduction based assayMore data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of human DHODH after 20 mins by 2,6-dichloroindophenol-reduction based assayMore data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of human DHODH after 20 mins by 2,6-dichloroindophenol-reduction based assayMore data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of human DHODH after 20 mins by 2,6-dichloroindophenol-reduction based assayMore data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of human DHODH after 20 mins by 2,6-dichloroindophenol-reduction based assayMore data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of human DHODH after 20 mins by 2,6-dichloroindophenol-reduction based assayMore data for this Ligand-Target Pair