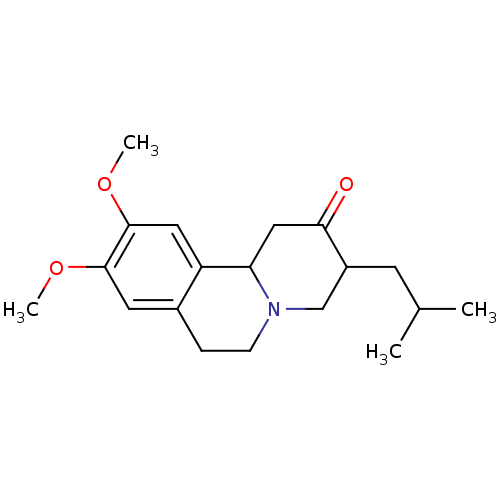
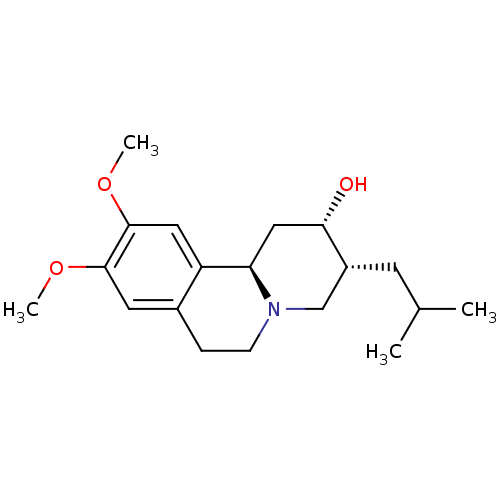
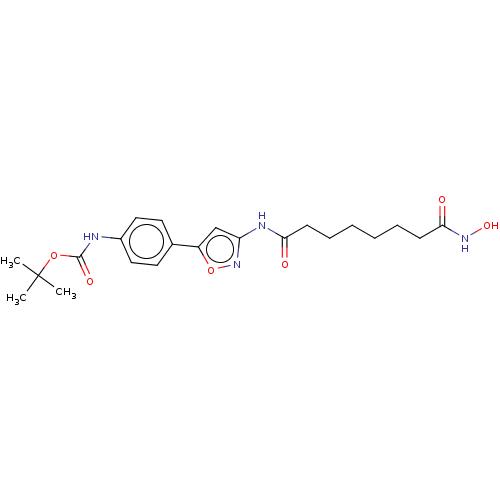
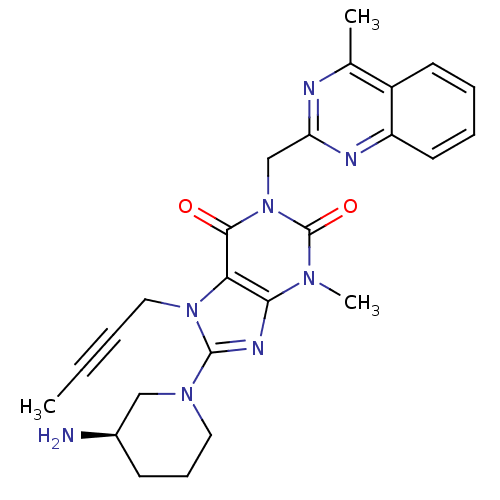
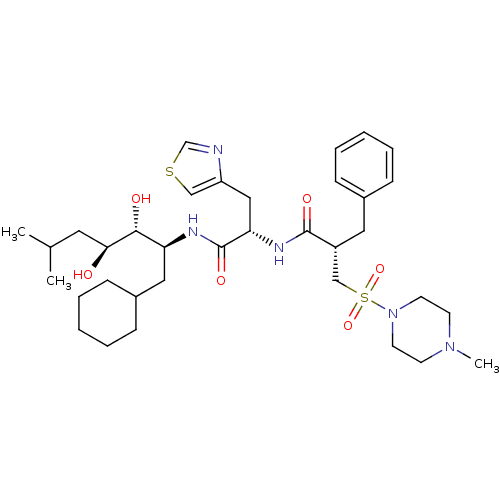
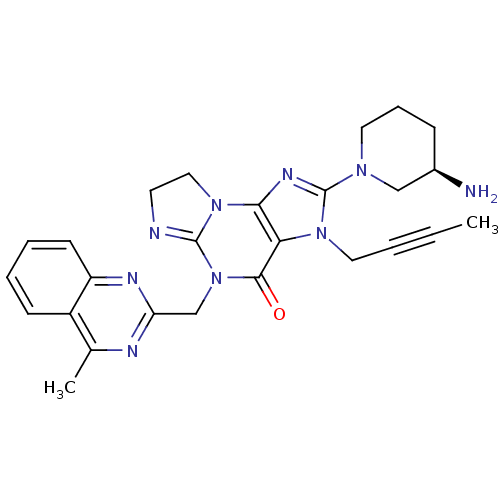
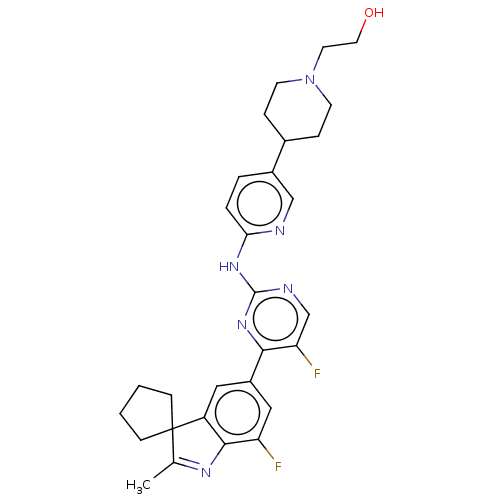
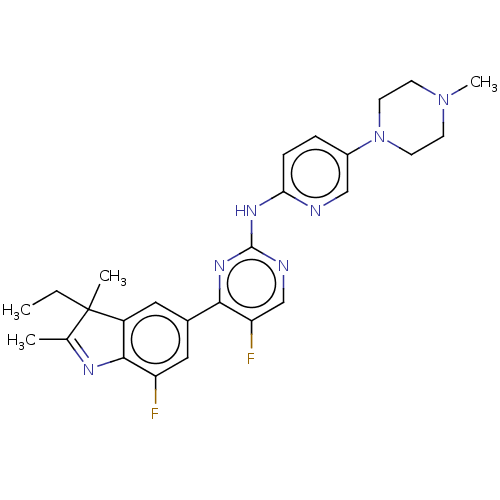
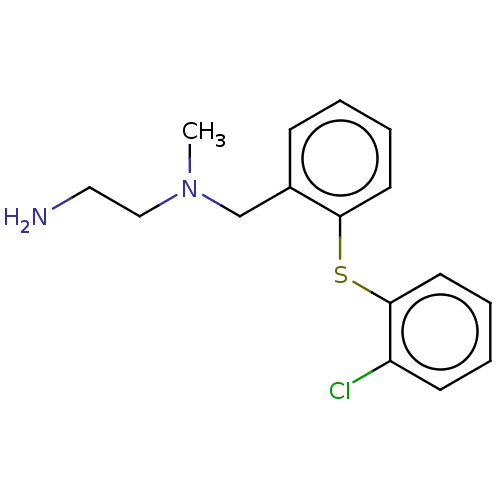
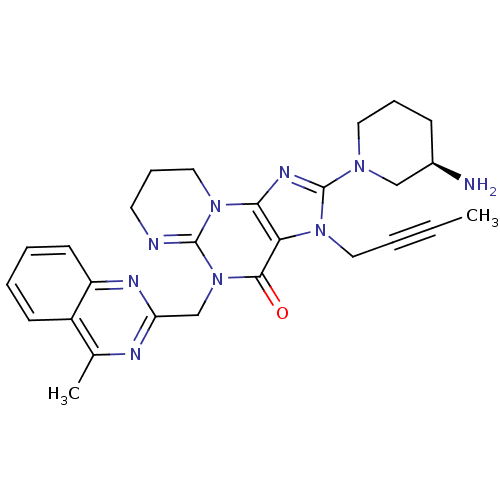
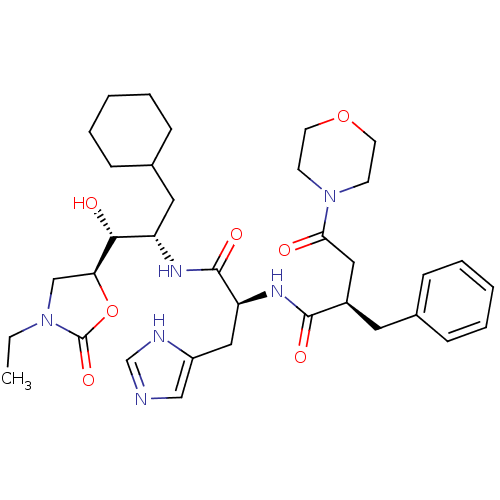
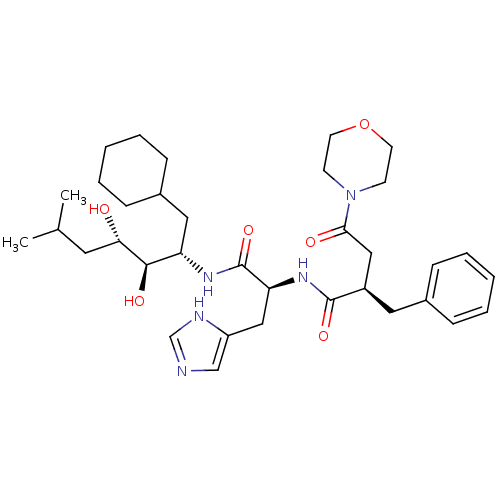
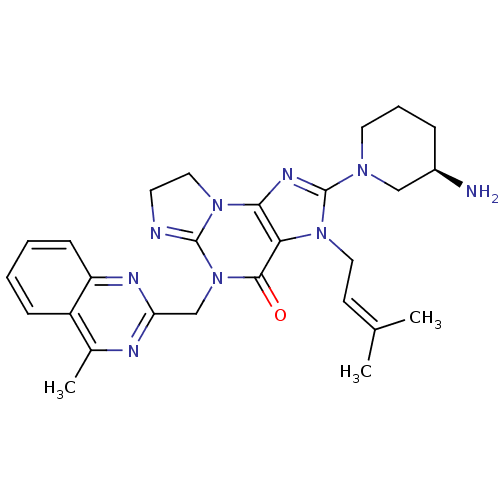
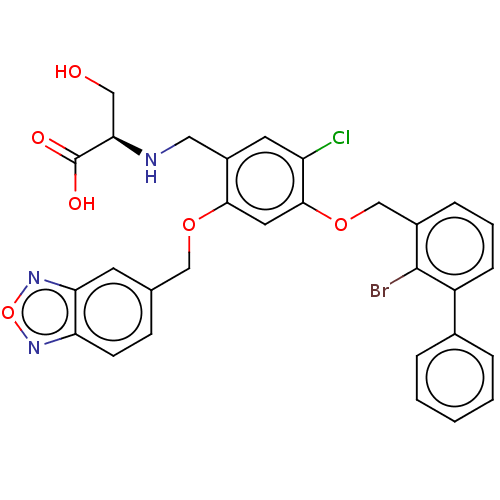
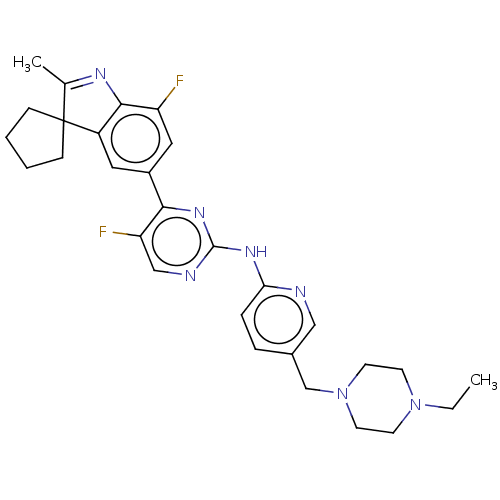
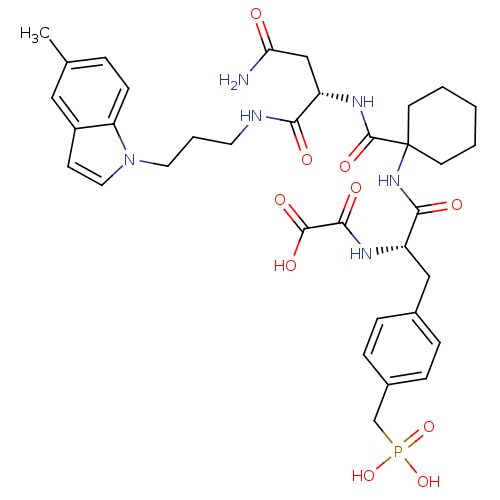
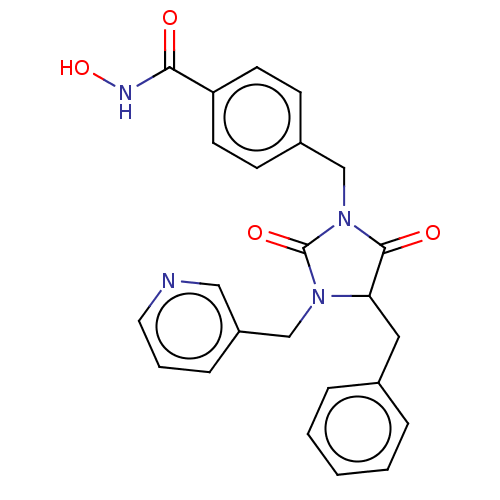
TargetSynaptic vesicular amine transporter(Rattus norvegicus (Rat))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataKi: 3.96nMAssay Description:Displacement of [3H]DHTBZ from Sprague-Dawley rat striatum VMAT2 after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
TargetSynaptic vesicular amine transporter(Rattus norvegicus (Rat))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataKi: 4.47nMAssay Description:Displacement of [3H]DHTBZ from Sprague-Dawley rat striatum VMAT2 after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
TargetSynaptic vesicular amine transporter(Rattus norvegicus (Rat))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataKi: 7.62nMAssay Description:Displacement of [3H]DHTBZ from Sprague-Dawley rat striatum VMAT2 after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
TargetSynaptic vesicular amine transporter(Rattus norvegicus (Rat))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataKi: 13.4nMAssay Description:Displacement of [3H]DHTBZ from Sprague-Dawley rat striatum VMAT2 after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
TargetSynaptic vesicular amine transporter(Rattus norvegicus (Rat))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataKi: 71.1nMAssay Description:Displacement of [3H]DHTBZ from Sprague-Dawley rat striatum VMAT2 after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
TargetSynaptic vesicular amine transporter(Rattus norvegicus (Rat))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataKi: 593nMAssay Description:Displacement of [3H]DHTBZ from Sprague-Dawley rat striatum VMAT2 after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
TargetSynaptic vesicular amine transporter(Rattus norvegicus (Rat))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataKi: 1.25E+3nMAssay Description:Displacement of [3H]DHTBZ from Sprague-Dawley rat striatum VMAT2 after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
TargetSynaptic vesicular amine transporter(Rattus norvegicus (Rat))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataKi: 2.46E+3nMAssay Description:Displacement of [3H]DHTBZ from Sprague-Dawley rat striatum VMAT2 after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
TargetSynaptic vesicular amine transporter(Rattus norvegicus (Rat))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataKi: 4.63E+3nMAssay Description:Displacement of [3H]DHTBZ from Sprague-Dawley rat striatum VMAT2 after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
TargetSynaptic vesicular amine transporter(Rattus norvegicus (Rat))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataKi: 2.37E+4nMAssay Description:Displacement of [3H]DHTBZ from Sprague-Dawley rat striatum VMAT2 after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
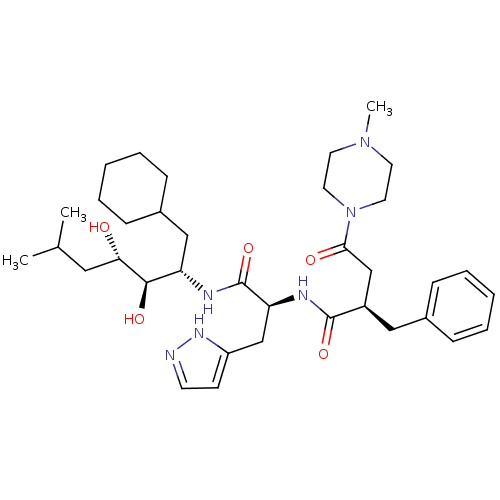
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 2.90E+4nMAssay Description:Inhibitory potency against human Protein-tyrosine phosphatase 1B expressed in E. coli BL21 (DE3) cellsMore data for this Ligand-Target Pair
TargetSynaptic vesicular amine transporter(Rattus norvegicus (Rat))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataKi: 3.64E+4nMAssay Description:Displacement of [3H]DHTBZ from Sprague-Dawley rat striatum VMAT2 after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.00200nMAssay Description:Inhibition of HDAC6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Inhibition of human DPP4 preincubated for 30 mins followed by Gly-Pro-AMC addition measured for 50 mins by continuous fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.240nMpH: 7.4Assay Description:In vitro inhibitory concentration against monkey plasma renin at pH 7.4More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 6/G1/S-specific cyclin-D3(Homo sapiens (Human))
Beijing Normal University
Curated by ChEMBL
Beijing Normal University
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of CDK6/Cyclin-D3 (unknown origin) using histoneH1 as substrate after 90 mins by ADP-Glo assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.420nMAssay Description:Inhibition of HDAC3 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.420nMAssay Description:Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 50 mins by ...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 6/G1/S-specific cyclin-D3(Homo sapiens (Human))
Beijing Normal University
Curated by ChEMBL
Beijing Normal University
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of CDK6/Cyclin-D3 (unknown origin) using histoneH1 as substrate after 90 mins by ADP-Glo assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.720nMpH: 7.4Assay Description:Inhibitory concentration against human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 0.760nMpH: 7.4Assay Description:In vitro inhibitory concentration against monkey plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMpH: 7.4Assay Description:Inhibitory concentration against human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 0.870nMpH: 7.4Assay Description:Inhibitory concentration against human plasma renin at pH 7.4More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 6/G1/S-specific cyclin-D3(Homo sapiens (Human))
Beijing Normal University
Curated by ChEMBL
Beijing Normal University
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of CDK6/Cyclin-D3 (unknown origin) using histoneH1 as substrate after 90 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 6/G1/S-specific cyclin-D3(Homo sapiens (Human))
Beijing Normal University
Curated by ChEMBL
Beijing Normal University
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of CDK6/Cyclin-D3 (unknown origin) using histoneH1 as substrate after 90 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetProtein arginine N-methyltransferase 8(Homo sapiens (Human))
Zhejiang Sci-Tech University
Curated by ChEMBL
Zhejiang Sci-Tech University
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibition of PRMT8 (unknown origin) incubated for 15 mins followed by substrate addition measured after 60 mins by AlphaLisa methodMore data for this Ligand-Target Pair
TargetProtein arginine N-methyltransferase 1(Rattus norvegicus)
Zhejiang Sci-Tech University
Curated by ChEMBL
Zhejiang Sci-Tech University
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibition of rat His-tagged PRMT1 (11 to 353 residues) expressed in Escherichia coli BL21(DE3) incubated for 15 mins followed by substrate addition ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMpH: 7.4Assay Description:In vitro inhibitory concentration against human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMpH: 7.4Assay Description:In vitro inhibitory concentration against human plasma renin at pH 7.4More data for this Ligand-Target Pair
TargetProtein arginine N-methyltransferase 6(Homo sapiens (Human))
Zhejiang Sci-Tech University
Curated by ChEMBL
Zhejiang Sci-Tech University
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of PRMT6 (unknown origin) incubated for 15 mins followed by substrate addition measured after 60 mins by AlphaLisa methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 50 mins by ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMpH: 7.4Assay Description:Inhibitory concentration against human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMpH: 7.4Assay Description:Inhibitory concentration against human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMpH: 7.4Assay Description:Inhibitory concentration against human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 50 mins by ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Inhibition of human DPP4 preincubated for 30 mins followed by Gly-Pro-AMC addition measured for 50 mins by continuous fluorescence assayMore data for this Ligand-Target Pair
TargetProgrammed cell death 1 ligand/protein 1(Homo sapiens)
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of human PD-1/PD-L1 interaction assessed as blockade activity incubated for 15 mins by HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMpH: 7.4Assay Description:Inhibitory concentration against human plasma renin at pH 7.4More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 6/G1/S-specific cyclin-D3(Homo sapiens (Human))
Beijing Normal University
Curated by ChEMBL
Beijing Normal University
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of CDK6/Cyclin-D3 (unknown origin) using histoneH1 as substrate after 90 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetGrowth factor receptor-bound protein 2(Homo sapiens (Human))
National Cancer Institute-Bethesda
Curated by ChEMBL
National Cancer Institute-Bethesda
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of Growth factor receptor bound protein 2 binding by ELISA assay methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Inhibition of HDAC6 (unknown origin) using Ac-LeuGlyLy-s(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition and further inc...More data for this Ligand-Target Pair
TargetProgrammed cell death 1 ligand/protein 1(Homo sapiens)
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 2.60nMAssay Description:Inhibition of human PD-1/PD-L1 interaction assessed as blockade activity incubated for 15 mins by HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMpH: 7.4Assay Description:In vitro inhibitory concentration against human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 4/G1/S-specific cyclin-D1(Homo sapiens (Human))
Beijing Normal University
Curated by ChEMBL
Beijing Normal University
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of CDK4/Cyclin-D1 (unknown origin) using histone-H1 as substrate after 90 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetMicrotubule-associated proteins 1A/1B light chain 3B(Human)
Wigen Biomedicine Technology (Shanghai)
US Patent
Wigen Biomedicine Technology (Shanghai)
US Patent
Affinity DataIC50: <3nMAssay Description:By constructing a prokaryotic expression system, the LC3B protein was successfully expressed and purified, and a preliminary screening and verificati...More data for this Ligand-Target Pair
TargetMicrotubule-associated proteins 1A/1B light chain 3B(Human)
Wigen Biomedicine Technology (Shanghai)
US Patent
Wigen Biomedicine Technology (Shanghai)
US Patent
Affinity DataIC50: <3nMAssay Description:By constructing a prokaryotic expression system, the LC3B protein was successfully expressed and purified, and a preliminary screening and verificati...More data for this Ligand-Target Pair
TargetMicrotubule-associated proteins 1A/1B light chain 3B(Human)
Wigen Biomedicine Technology (Shanghai)
US Patent
Wigen Biomedicine Technology (Shanghai)
US Patent
Affinity DataIC50: <3nMAssay Description:By constructing a prokaryotic expression system, the LC3B protein was successfully expressed and purified, and a preliminary screening and verificati...More data for this Ligand-Target Pair

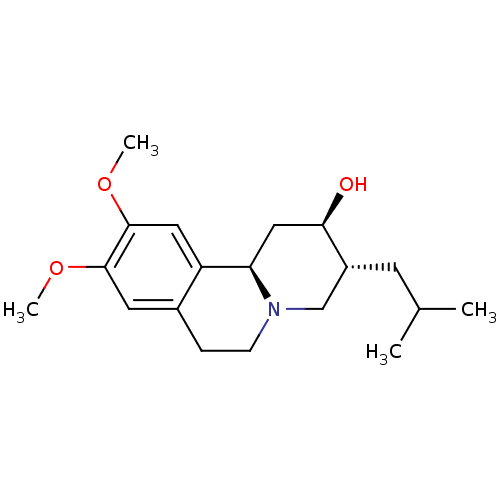
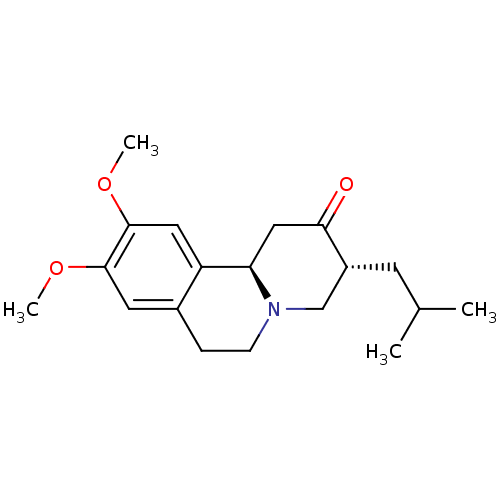
 3D Structure (crystal)
3D Structure (crystal)