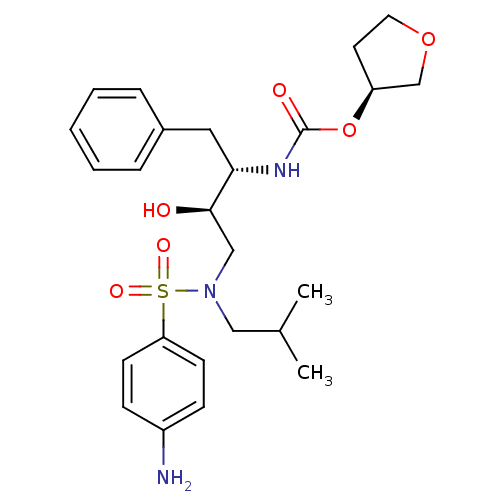
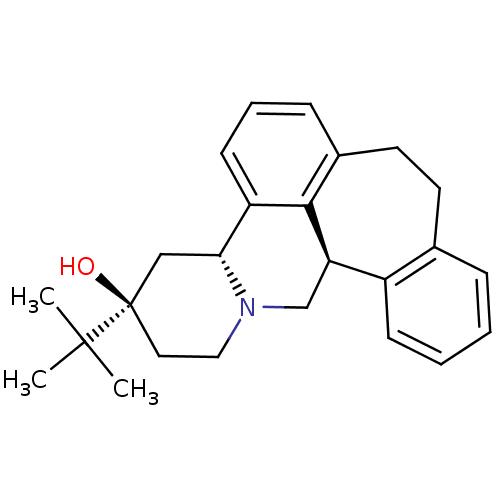
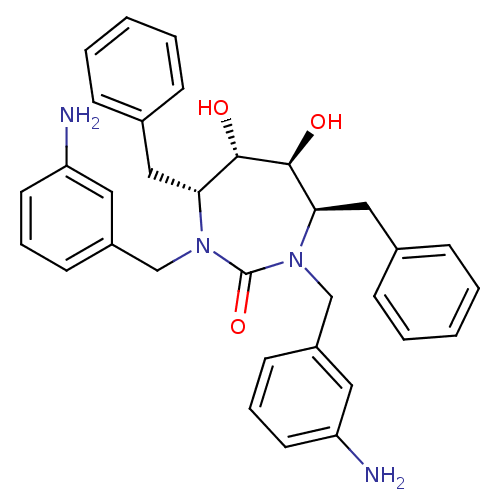
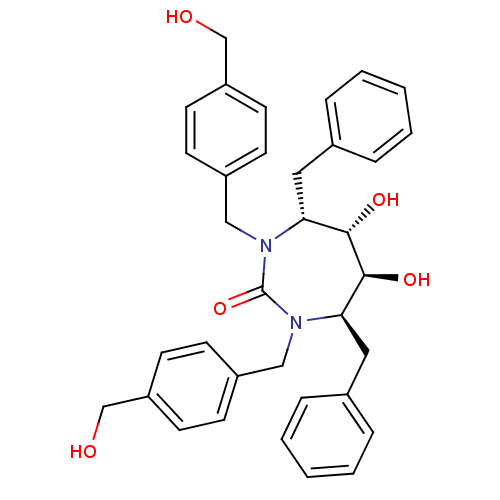
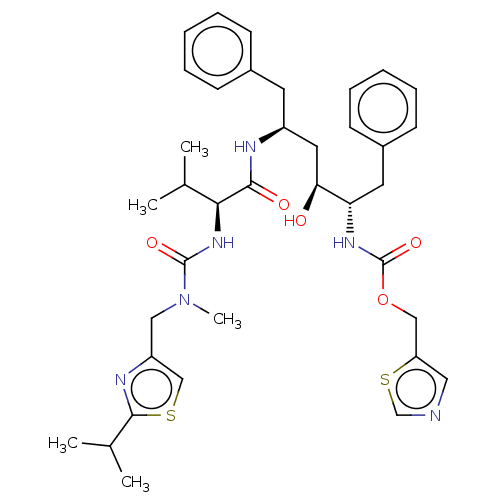
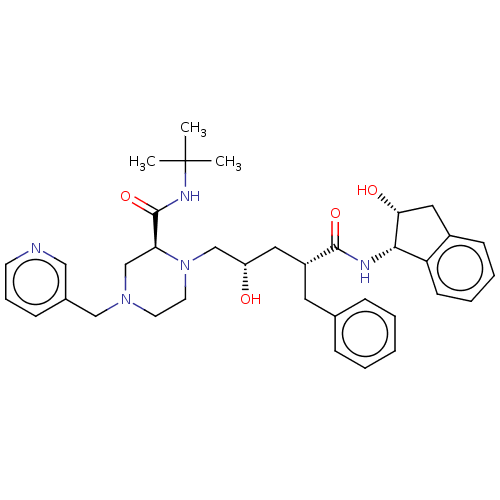
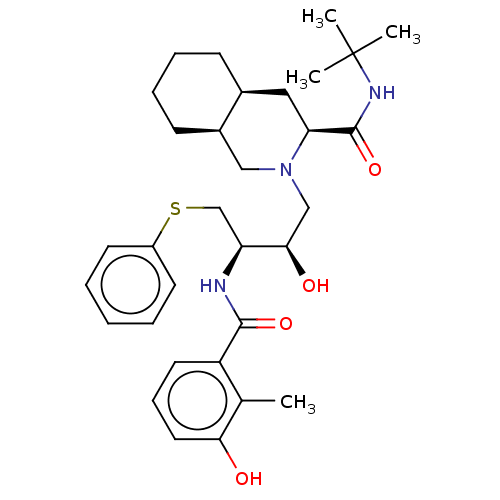
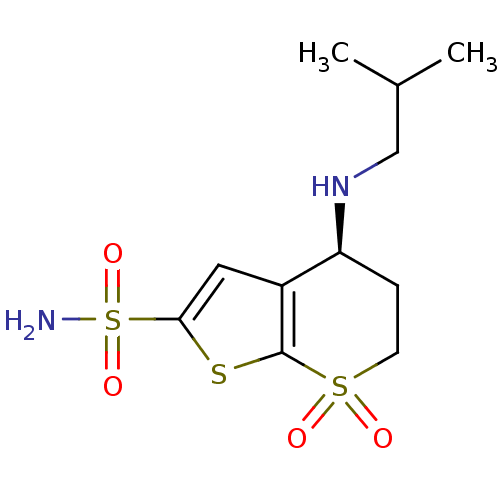
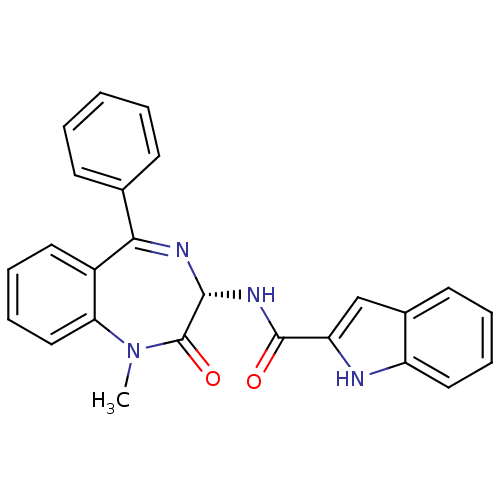
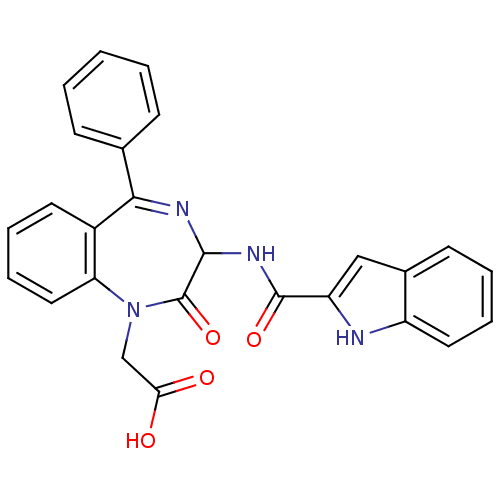
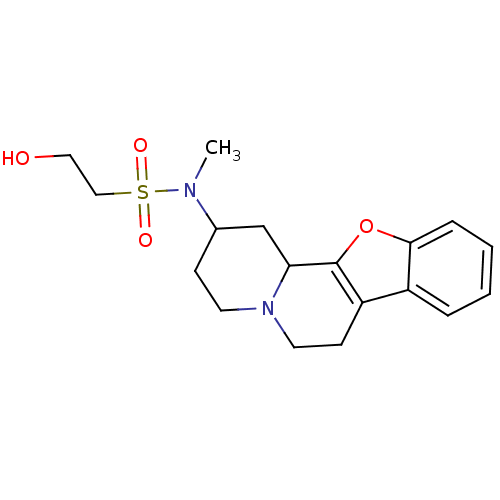
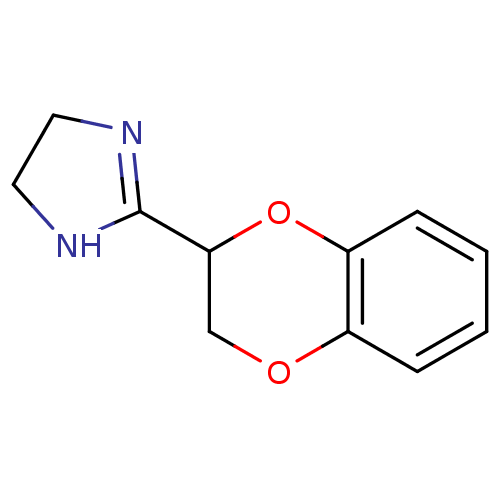
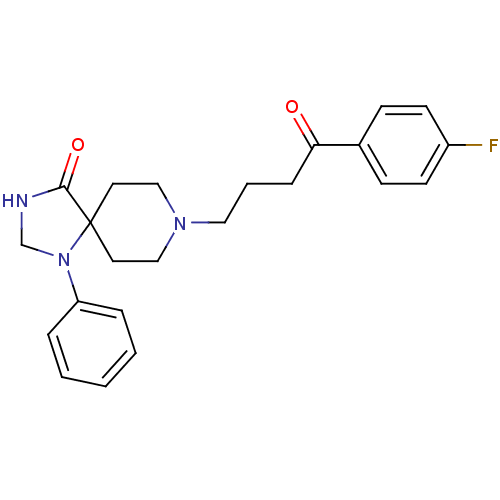
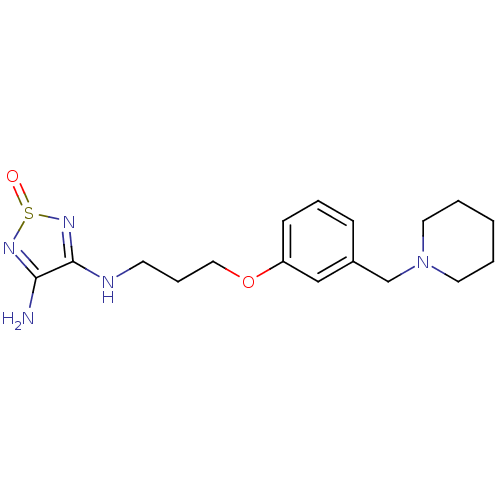
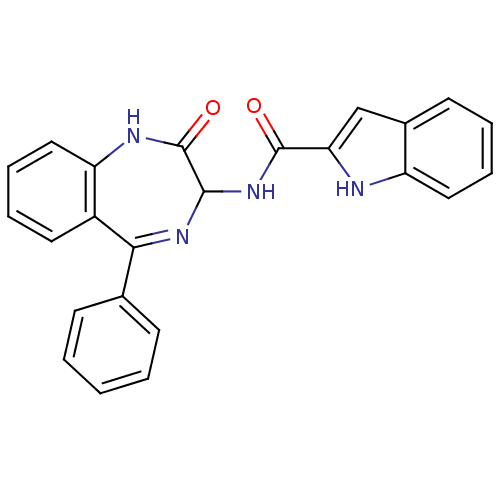
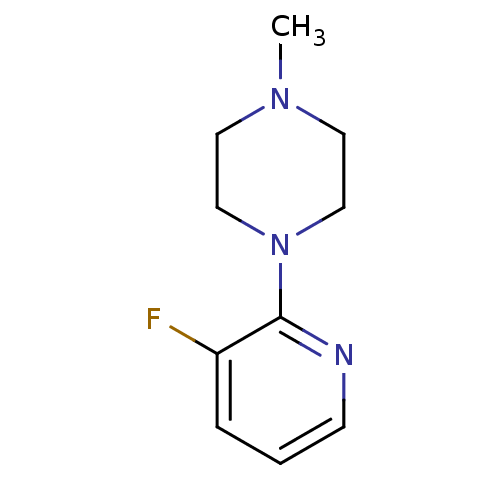
Affinity DataKi: <0.0100nMpH: 5.5Assay Description:Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease.More data for this Ligand-Target Pair
Affinity DataKi: 0.0100nMpH: 5.5Assay Description:Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease.More data for this Ligand-Target Pair
Affinity DataKi: 0.0160nMpH: 5.5Assay Description:Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease.More data for this Ligand-Target Pair
Affinity DataKi: 0.0180nMpH: 5.5Assay Description:Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease.More data for this Ligand-Target Pair
Affinity DataKi: 0.0180nMpH: 5.5Assay Description:Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease.More data for this Ligand-Target Pair
Affinity DataKi: 0.0200nMpH: 5.5Assay Description:Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease.More data for this Ligand-Target Pair
Affinity DataKi: 0.0210nMpH: 5.5Assay Description:Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease.More data for this Ligand-Target Pair
Affinity DataKi: 0.0230nMpH: 5.5Assay Description:Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease.More data for this Ligand-Target Pair
Affinity DataKi: 0.0310nMpH: 5.5Assay Description:Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease.More data for this Ligand-Target Pair
Affinity DataKi: 0.0410nMpH: 5.5Assay Description:Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease.More data for this Ligand-Target Pair
Affinity DataKi: 0.0410nMpH: 5.5Assay Description:Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease.More data for this Ligand-Target Pair
Affinity DataKi: 0.0900nMpH: 5.5Assay Description:Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease.More data for this Ligand-Target Pair
Affinity DataKi: 0.130nMpH: 5.5Assay Description:Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease.More data for this Ligand-Target Pair
Affinity DataKi: 0.150nMpH: 5.5Assay Description:Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease.More data for this Ligand-Target Pair
Affinity DataKi: 0.170nMpH: 5.5Assay Description:Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease.More data for this Ligand-Target Pair
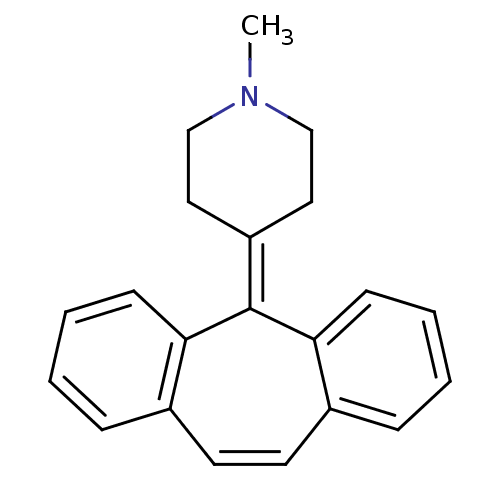
Affinity DataKi: 0.200nMAssay Description:Displacement of [3H]- spiperone radioligand binding at the dopamine binding site of rat caudateMore data for this Ligand-Target Pair
Affinity DataKi: 0.230nMpH: 5.5Assay Description:Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease.More data for this Ligand-Target Pair
Affinity DataKi: 0.300nMpH: 5.5Assay Description:Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease.More data for this Ligand-Target Pair
Affinity DataKi: 0.310nMpH: 5.5Assay Description:Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease.More data for this Ligand-Target Pair
Affinity DataKi: 0.340nMpH: 5.5Assay Description:Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease.More data for this Ligand-Target Pair
Affinity DataKi: 0.370nMpH: 5.5Assay Description:Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease.More data for this Ligand-Target Pair
Affinity DataKi: 0.370nMpH: 5.5Assay Description:Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease.More data for this Ligand-Target Pair
Affinity DataKi: 0.530nMpH: 5.5Assay Description:Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease.More data for this Ligand-Target Pair
Affinity DataKi: 0.690nMAssay Description:The equilibrium dissociation constant of the inhibitor-enzyme complex of human Carbonic anhydrase IIMore data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:50% inhibitory concentration against human carbonic anhydrase II (HCA II) after pre-incubation for 4 min at 37 degree CMore data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:The equilibrium dissociation constant of the inhibitor-enzyme complex of human Carbonic anhydrase IIMore data for this Ligand-Target Pair
Affinity DataKi: 0.770nMAssay Description:Evaluated for its ability to displace [3H]-clonidine from alpha-2 adrenergic receptor of calf cerebral cortexMore data for this Ligand-Target Pair
Affinity DataKi: 0.820nMAssay Description:50% inhibitory concentration against human carbonic anhydrase II (HCA II) after pre-incubation at 3 degree CMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:The equilibrium dissociation constant of the inhibitor-enzyme complex of human Carbonic anhydrase IIMore data for this Ligand-Target Pair
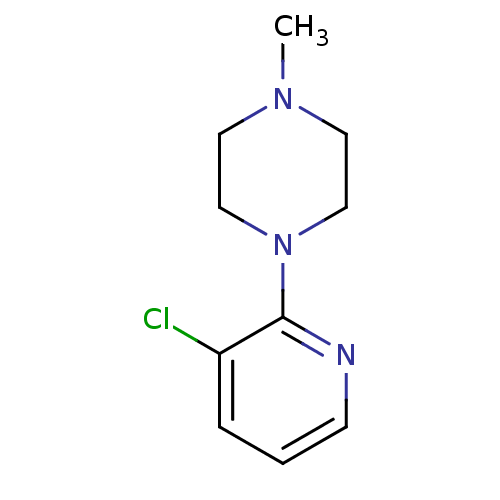
TargetAlpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor(RAT)
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 1.10nMAssay Description:Compound was tested for inhibition of human plasma reninMore data for this Ligand-Target Pair
Affinity DataKi: 1.30nMpH: 5.5Assay Description:Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease.More data for this Ligand-Target Pair
Affinity DataKi: 1.40nMAssay Description:Evaluated for its ability to displace [3H]-clonidine from alpha-2 adrenergic receptor of calf cerebral cortexMore data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Binding activity against alpha-2 adrenergic receptor from calf cerebral cortex, using [3H]-clonidine as the radioligandMore data for this Ligand-Target Pair
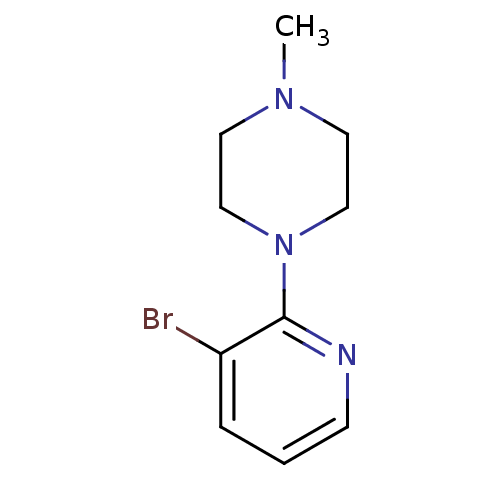
TargetAlpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor(RAT)
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 1.60nMAssay Description:Compound was tested for inhibition of hog kidney reninMore data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:The equilibrium dissociation constant of the inhibitor-enzyme complex of human Carbonic anhydrase IIMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Displacement of [3H]- spiperone radioligand binding at the dopamine binding site of rat caudateMore data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:The equilibrium dissociation constant of the inhibitor-enzyme complex of human Carbonic anhydrase IIMore data for this Ligand-Target Pair
Affinity DataKi: 2.70nMAssay Description:Binding activity against alpha-2 adrenergic receptor from calf cerebral cortex, using [3H]-clonidine as the radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 2.90nMAssay Description:Binding activity against alpha-2 adrenergic receptor from calf cerebral cortex, using [3H]-clonidine as the radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 3.70nMAssay Description:The equilibrium dissociation constant of the inhibitor-enzyme complex of human Carbonic anhydrase IIMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Displacement of [3H]- QNB binding at the muscarinic-cholinergic binding site of rat brain S1More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:In vitro inhibition of Histamine H2 receptor by measuring its ability to block the histamine-stimulated adenylate cyclase of guinea pig hippocampal h...More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Binding activity against alpha-2 adrenergic receptor from calf cerebral cortex, using [3H]-clonidine as the radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 6.20nMAssay Description:50% inhibitory concentration against human carbonic anhydrase II (HCA II) after pre-incubation for 4 min at 37 degree CMore data for this Ligand-Target Pair

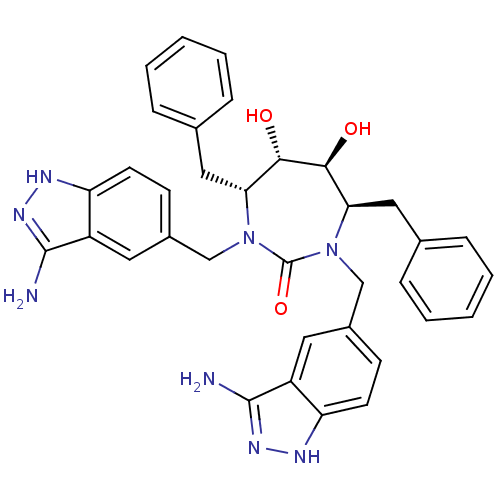
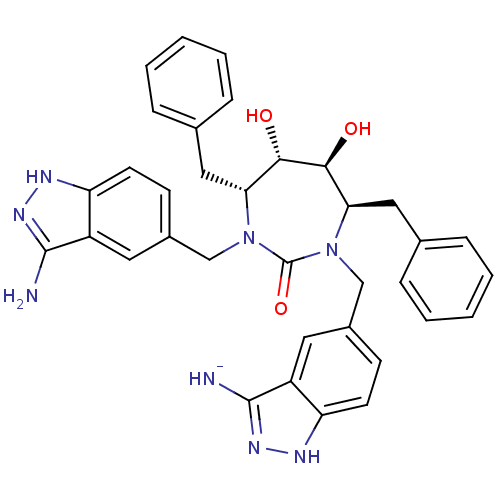
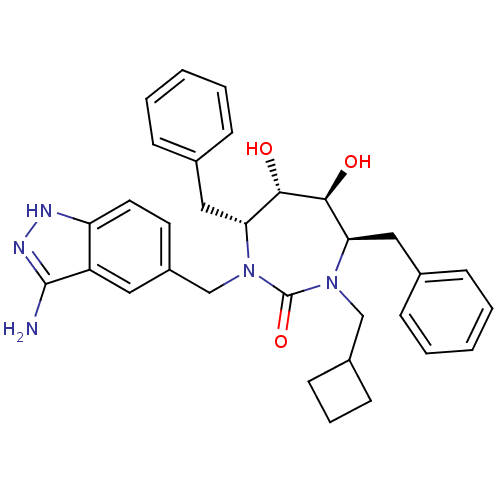

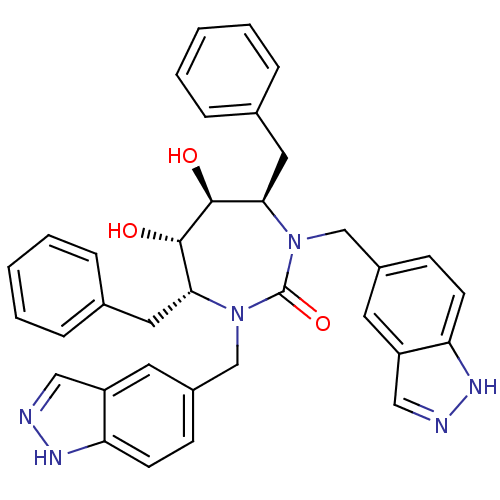


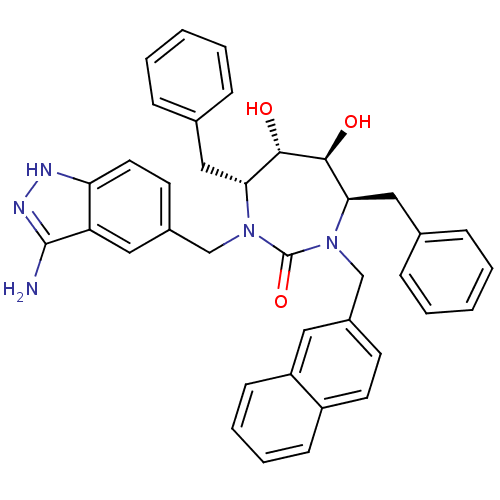




 3D Structure (crystal)
3D Structure (crystal)