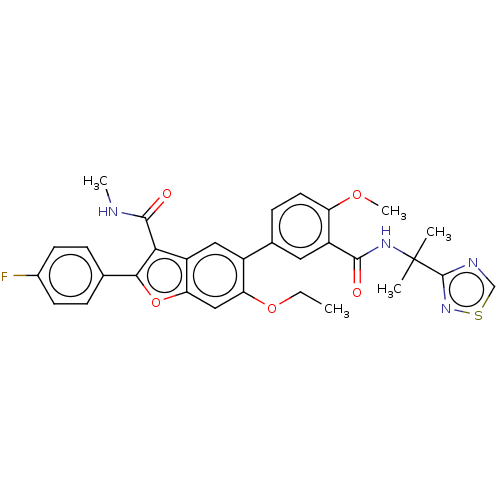
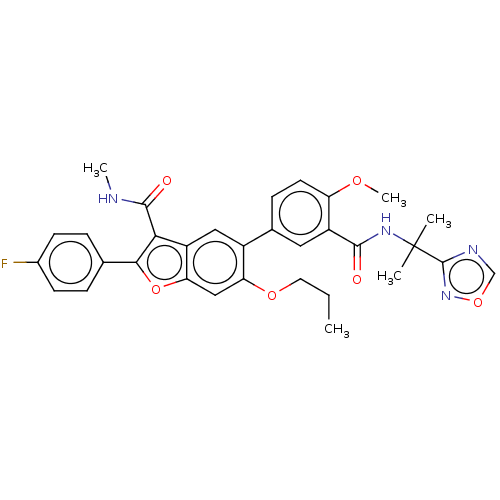
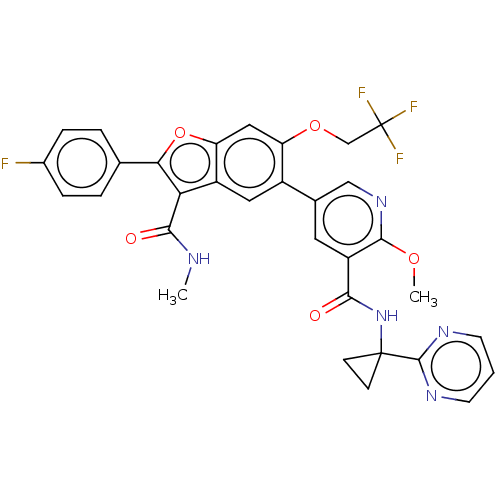
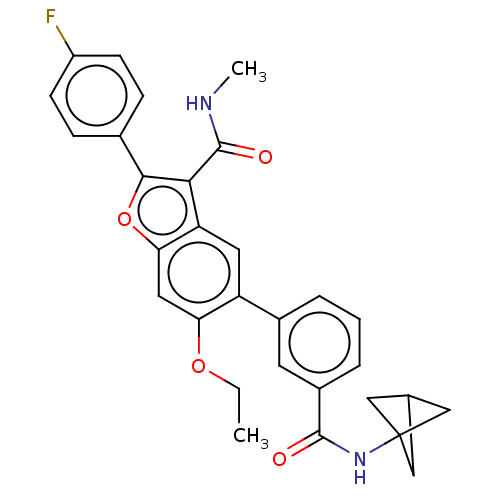
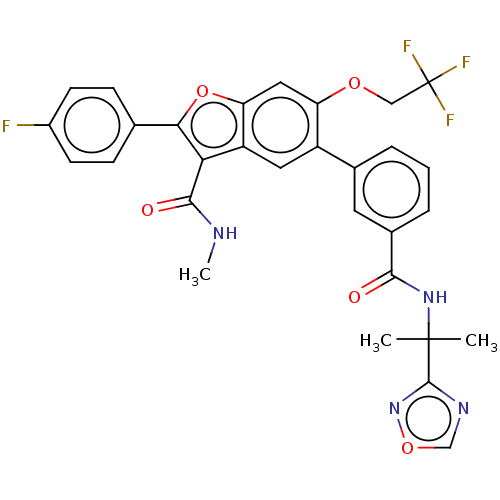
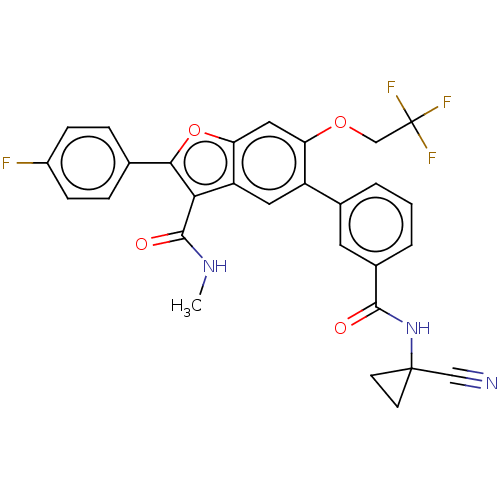
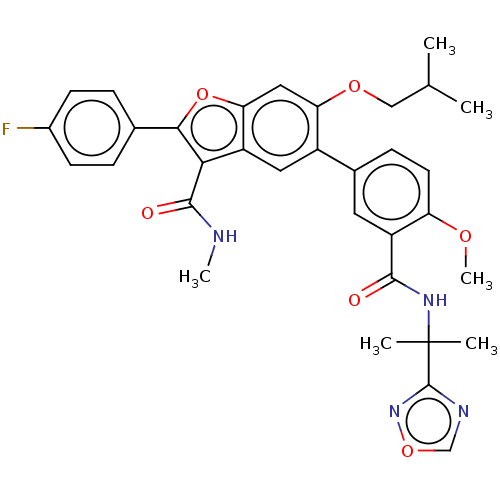
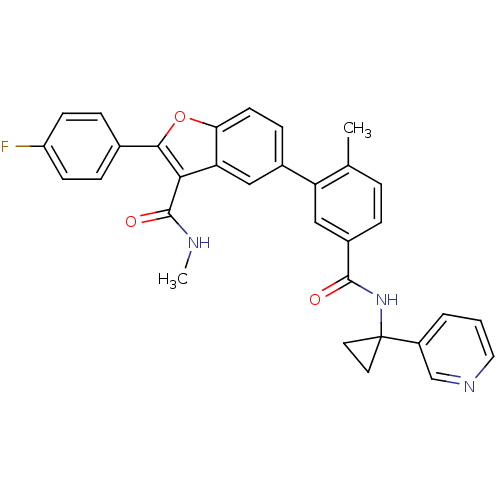
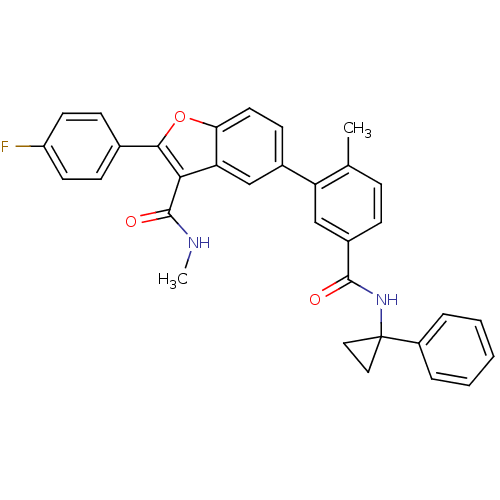
Affinity DataIC50: 0.603nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Antagonist activity at CCR2 in human THP-1 cells assessed as reduction in CCL2-induced chemotaxisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Antagonist activity at CCR2 in human THP-1 cells assessed as reduction in CCL2-induced chemotaxisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Antagonist activity at CCR2 in human THP-1 cells assessed as reduction in CCL2-induced chemotaxisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Antagonist activity at CCR5 in human peripheral T cells assessed as reduction in MIP-1beta-induced chemotaxisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Antagonist activity at CCR5 in human peripheral T cells assessed as reduction in MIP-1beta-induced chemotaxisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.52nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair
Affinity DataIC50: 1.84nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair
Affinity DataIC50: 1.89nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair
Affinity DataIC50: 2.04nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair
Affinity DataIC50: 2.04nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair
Affinity DataIC50: 2.22nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair
Affinity DataIC50: 2.48nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair
Affinity DataIC50: 2.48nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMAssay Description:Antagonist activity at CCR2 in human whole blood assessed as inhibition of CCL2-induced CD11b upregulationMore data for this Ligand-Target Pair
Affinity DataIC50: 2.72nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair
Affinity DataIC50: 2.73nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair
Affinity DataIC50: 2.83nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair
Affinity DataIC50: 2.95nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair
Affinity DataIC50: 3.02nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair
Affinity DataIC50: 3.31nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair
Affinity DataIC50: 3.60nMAssay Description:Displacement of [125I]MIP-1beta from CCR5 in human peripheral T cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.88nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair
Affinity DataIC50: 3.89nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair
Affinity DataIC50: 4.15nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair
Affinity DataIC50: 4.20nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair
Affinity DataIC50: 4.30nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair
Affinity DataIC50: 4.38nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair
Affinity DataIC50: 4.49nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair
Affinity DataIC50: 4.54nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair
Affinity DataIC50: 4.80nMAssay Description:Antagonist activity at CCR2 in human whole blood assessed as inhibition of CCL2-induced CD11b upregulationMore data for this Ligand-Target Pair
Affinity DataIC50: 4.97nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair
Affinity DataIC50: 5.05nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair
Affinity DataIC50: 5.70nMAssay Description:Antagonist activity at CCR5 in human whole blood assessed as inhibition of MIP-1beta-induced CD11b upregulationMore data for this Ligand-Target Pair
Affinity DataIC50: 5.78nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair
Affinity DataIC50: 6.01nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair
Affinity DataIC50: 6.20nMAssay Description:Displacement of [125I]-CCL2 from CCR2 in human PBMC cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 7.14nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair
Affinity DataIC50: 7.42nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair
TargetCytochrome P450 2C8(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 26nMAssay Description:Inhibition of microsomal CYP2C8 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair
Affinity DataIC50: 35nMAssay Description:Antagonist activity at CCR5 in human whole blood assessed as inhibition of MIP-1beta-induced CD11b upregulationMore data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Displacement of [125I]-CCL2 from mouse CCR2More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 63nMAssay Description:Inhibition of microsomal CYP3A4 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 73nMAssay Description:Inhibition of microsomal CYP3A4 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2C8(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 350nMAssay Description:Inhibition of microsomal CYP2C8 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2C8(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of microsomal CYP2C8 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2C8(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of microsomal CYP2C8 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of microsomal CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >2.50E+3nMAssay Description:To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with...More data for this Ligand-Target Pair