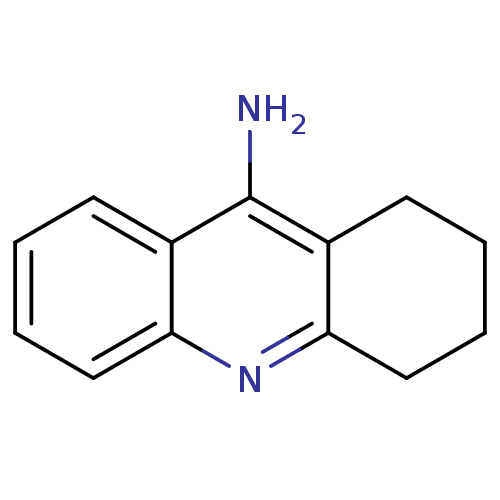
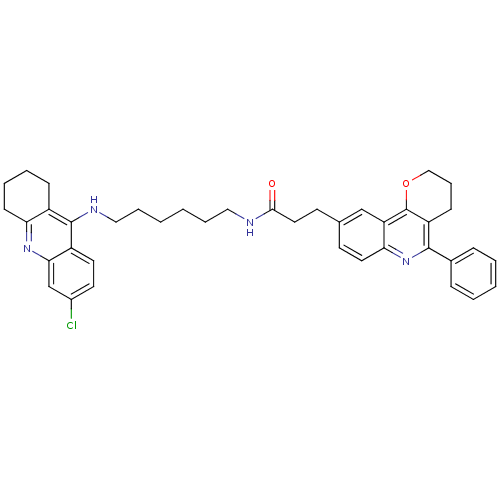
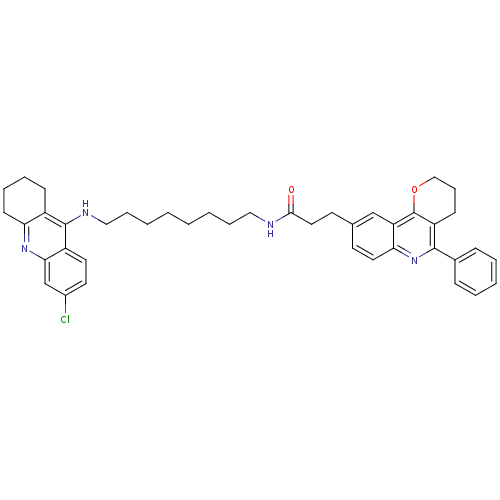
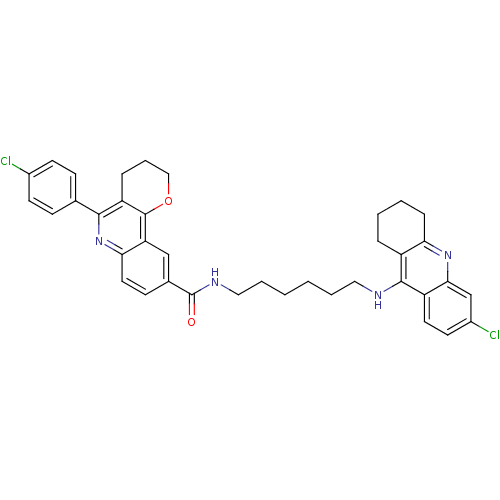
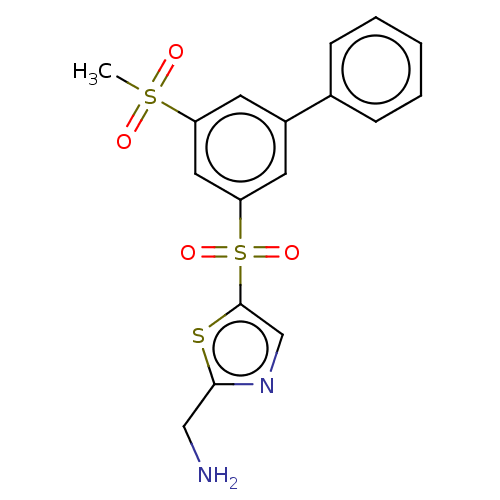
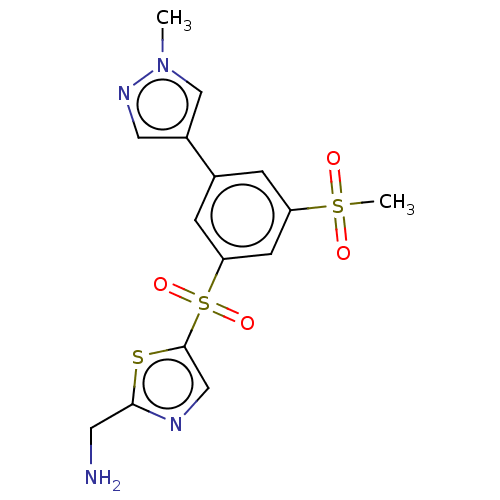
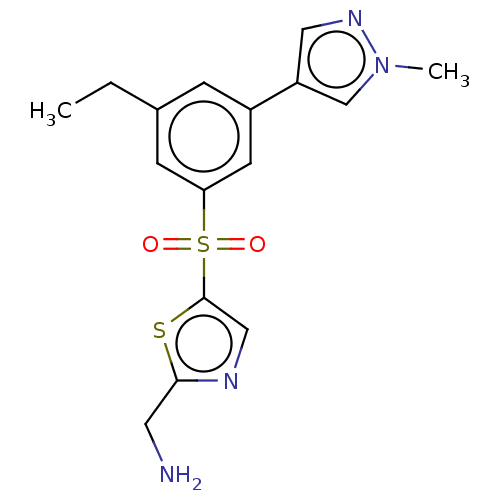
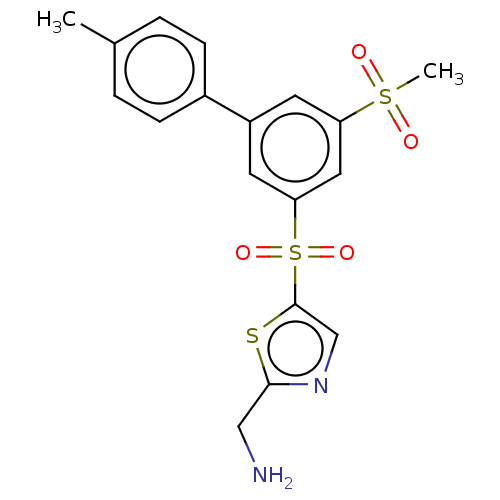
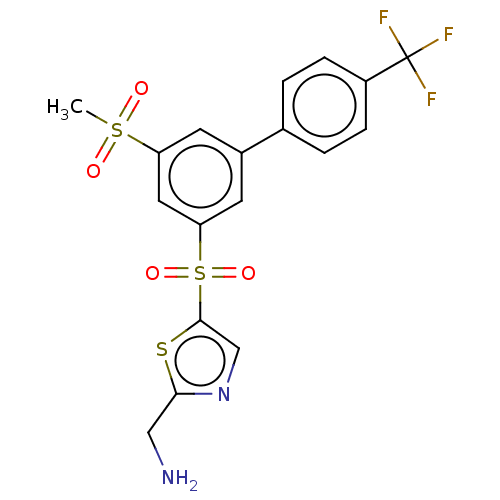
Affinity DataIC50: 5.73nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho...More data for this Ligand-Target Pair
Affinity DataIC50: 7.03nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho...More data for this Ligand-Target Pair
Affinity DataIC50: 8.32nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho...More data for this Ligand-Target Pair
Affinity DataIC50: 9.64nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of recombinant human AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrat...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of horse serum BuChE using butyrylthiocholine as substrate by UV spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of BuChE in horse serum by Ellman methodMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of equine serum BuChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of BuChE in horse serum using butyrylthiocholine as substrate by Ellman methodMore data for this Ligand-Target Pair
Affinity DataIC50: 10.3nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho...More data for this Ligand-Target Pair
Affinity DataIC50: 10.4nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho...More data for this Ligand-Target Pair
Affinity DataIC50: 11.1nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho...More data for this Ligand-Target Pair
Affinity DataIC50: 14.4nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho...More data for this Ligand-Target Pair
Affinity DataIC50: 16.6nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho...More data for this Ligand-Target Pair
Affinity DataIC50: 18.3nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho...More data for this Ligand-Target Pair
Affinity DataIC50: 19.2nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho...More data for this Ligand-Target Pair
Affinity DataIC50: 20.4nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho...More data for this Ligand-Target Pair
Affinity DataIC50: 23.9nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho...More data for this Ligand-Target Pair
Affinity DataIC50: 24nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho...More data for this Ligand-Target Pair
Affinity DataIC50: 24.9nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho...More data for this Ligand-Target Pair
Affinity DataIC50: 29.9nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho...More data for this Ligand-Target Pair
Affinity DataIC50: 30.8nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho...More data for this Ligand-Target Pair
Affinity DataIC50: 34.1nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho...More data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of AChE in bovine erythrocyte by Ellman methodMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of bovine erythrocyte AChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of AChE in bovine erythrocytes using acetylthiocholine as substrate by Ellman methodMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of human BuChE in serumMore data for this Ligand-Target Pair
Affinity DataIC50: 48.1nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho...More data for this Ligand-Target Pair
Affinity DataIC50: 50nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho...More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of BuChE in horse serum by Ellman methodMore data for this Ligand-Target Pair
Affinity DataIC50: 69nMAssay Description:Inhibition of human C-terminal His10-tagged LOXL2 (Met1 to Gln774 residues) expressed in mouse NS0 cells using cadaverine hydrochloride as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 69nMAssay Description:Inhibition of human C-terminal His10-tagged LOXL2 (Met1 to Gln774 residues) expressed in mouse NS0 cells using cadaverine hydrochloride as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 70nMAssay Description:Inhibition of human BuChE in serumMore data for this Ligand-Target Pair
Affinity DataIC50: 78nMAssay Description:Inhibition of human C-terminal His10-tagged LOXL2 (Met1 to Gln774 residues) expressed in mouse NS0 cells using cadaverine hydrochloride as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 85nMAssay Description:Inhibition of human C-terminal His10-tagged LOXL2 (Met1 to Gln774 residues) expressed in mouse NS0 cells using cadaverine hydrochloride as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 86nMAssay Description:Inhibition of human C-terminal His10-tagged LOXL2 (Met1 to Gln774 residues) expressed in mouse NS0 cells using cadaverine hydrochloride as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 93.7nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of human AChE in erythrocyteMore data for this Ligand-Target Pair
Affinity DataIC50: 103nMAssay Description:Inhibition of human C-terminal His10-tagged LOXL2 (Met1 to Gln774 residues) expressed in mouse NS0 cells using cadaverine hydrochloride as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 109nMAssay Description:Inhibition of human C-terminal His10-tagged LOXL2 (Met1 to Gln774 residues) expressed in mouse NS0 cells using cadaverine hydrochloride as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 120nMAssay Description:Inhibition of human C-terminal His10-tagged LOXL2 (Met1 to Gln774 residues) expressed in mouse NS0 cells using cadaverine hydrochloride as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 126nMAssay Description:Inhibition of human C-terminal His10-tagged LOXL2 (Met1 to Gln774 residues) expressed in mouse NS0 cells using cadaverine hydrochloride as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 143nMAssay Description:Inhibition of human C-terminal His10-tagged LOXL2 (Met1 to Gln774 residues) expressed in mouse NS0 cells using cadaverine hydrochloride as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 146nMAssay Description:Inhibition of human C-terminal His10-tagged LOXL2 (Met1 to Gln774 residues) expressed in mouse NS0 cells using cadaverine hydrochloride as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 151nMAssay Description:Inhibition of human C-terminal His10-tagged LOXL2 (Met1 to Gln774 residues) expressed in mouse NS0 cells using cadaverine hydrochloride as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 170nMAssay Description:Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured for 5 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 174nMAssay Description:Inhibition of human C-terminal His10-tagged LOXL2 (Met1 to Gln774 residues) expressed in mouse NS0 cells using cadaverine hydrochloride as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 176nMAssay Description:Inhibition of human C-terminal His10-tagged LOXL2 (Met1 to Gln774 residues) expressed in mouse NS0 cells using cadaverine hydrochloride as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 176nMAssay Description:Inhibition of human C-terminal His10-tagged LOXL2 (Met1 to Gln774 residues) expressed in mouse NS0 cells using cadaverine hydrochloride as substrate ...More data for this Ligand-Target Pair

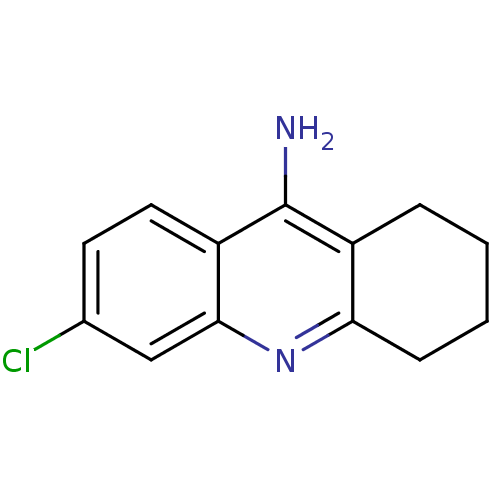


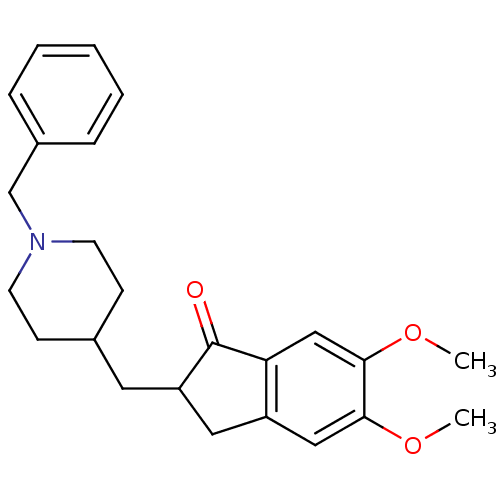
 3D Structure (crystal)
3D Structure (crystal)