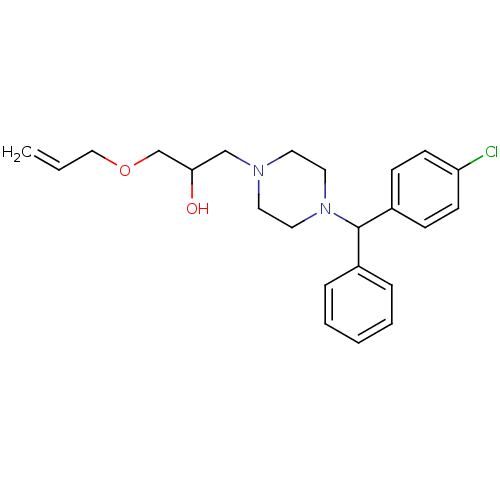
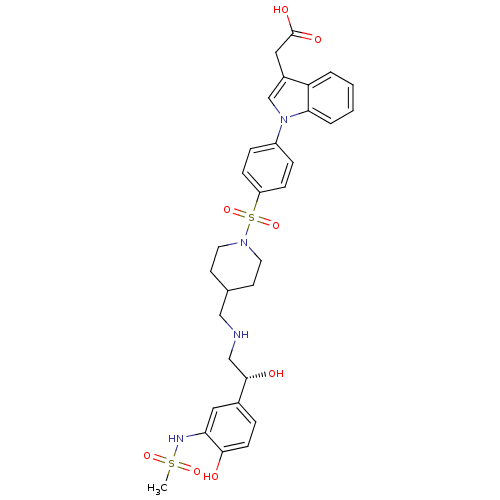
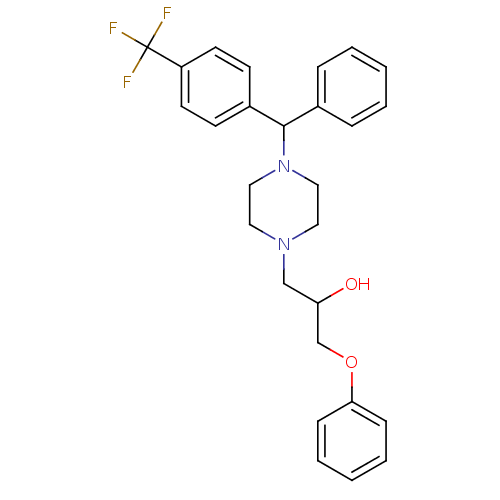
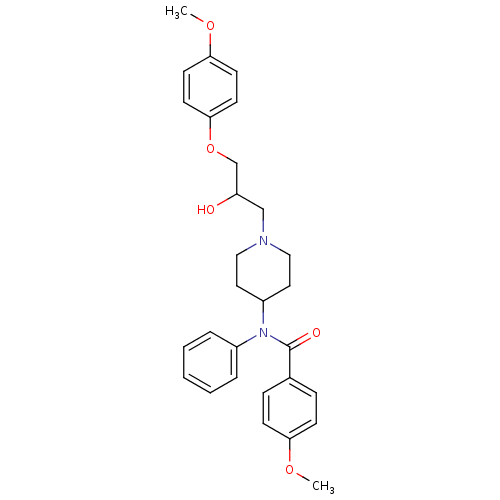
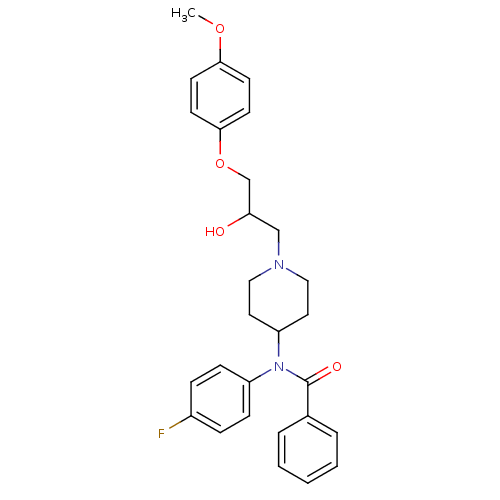
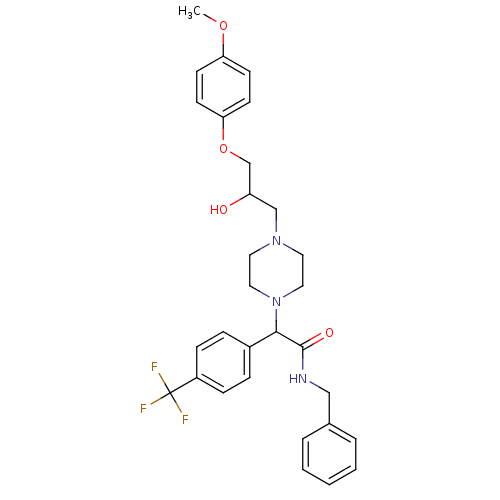
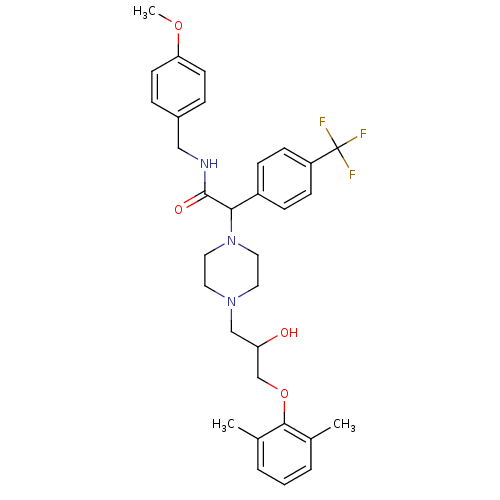
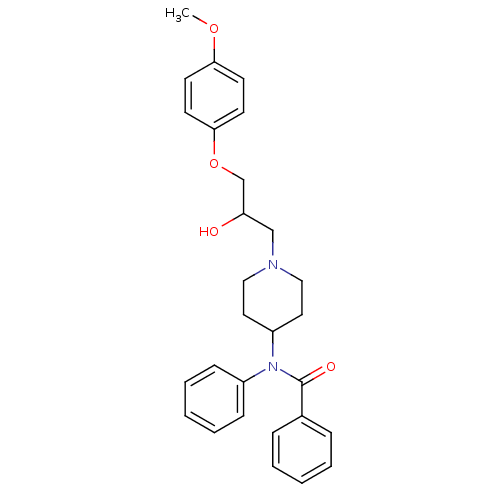
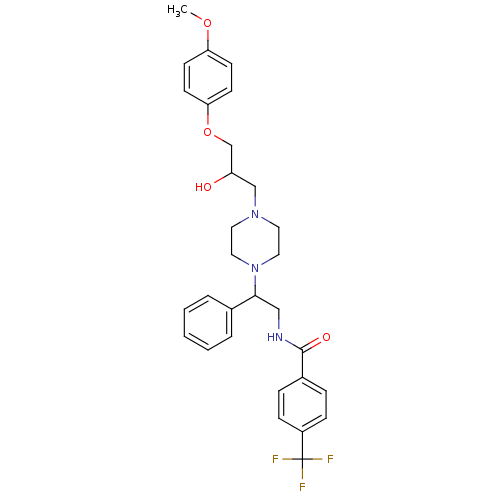
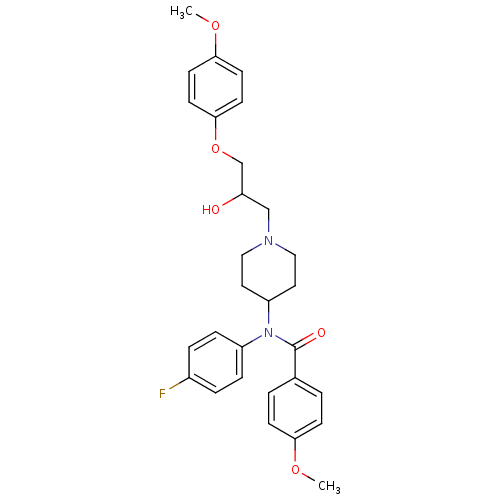
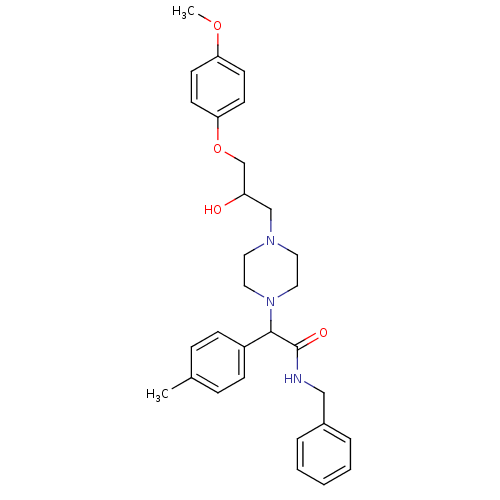
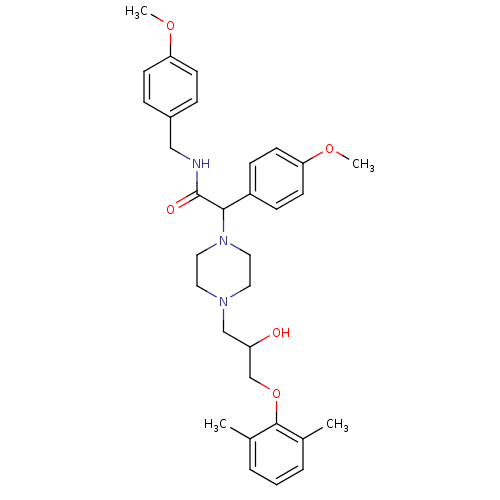
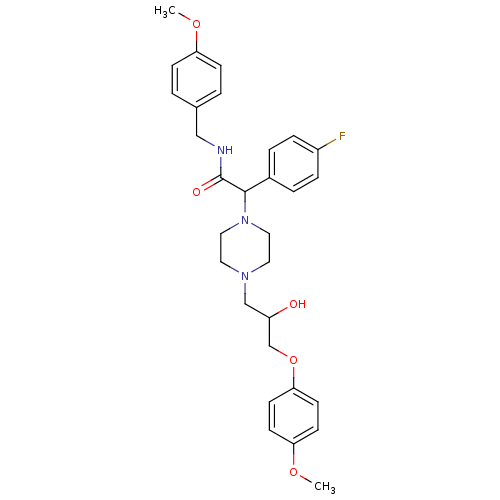
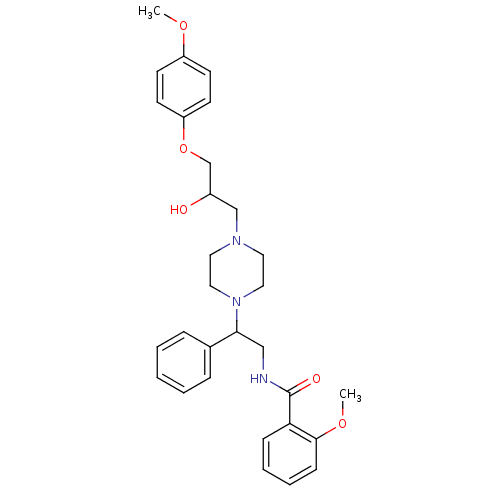
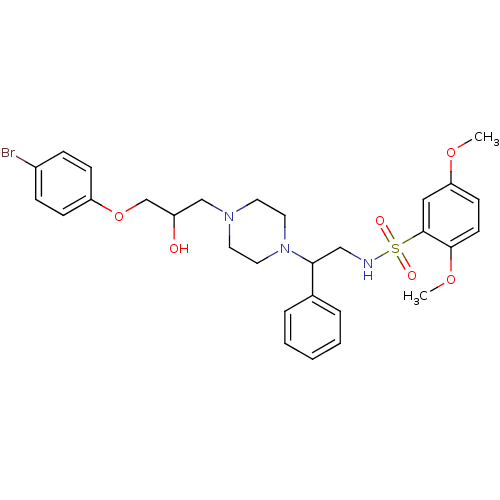
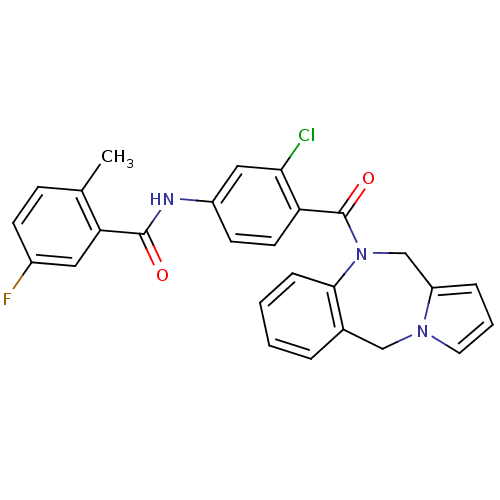
Affinity DataKi: 100nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 140nMAssay Description:Binding affinity of compound against Beta-2 adrenergic receptor was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 180nMAssay Description:Binding affinity of compound against Beta-2 adrenergic receptor was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 240nMAssay Description:Binding affinity of compound against Beta-1 adrenergic receptor was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 600nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 700nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 3.00E+3nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 3.00E+3nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 3.00E+3nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 3.00E+3nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 3.00E+3nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 4.00E+3nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 4.00E+3nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 4.00E+3nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 4.00E+3nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 5.00E+3nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 5.00E+3nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 6.60E+3nMAssay Description:Binding affinity of compound against Beta-1 adrenergic receptor was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 7.00E+3nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 7.00E+3nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 7.00E+3nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 8.00E+3nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 8.00E+3nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+4nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+4nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataKi: 3.00E+4nMAssay Description:Predictive competitive inhibition of cytochrome P450 2D6 was determined in silicoMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Displacement of [3H]-AVP from human vasopressin V2-receptor expressed in murine fibroblast cell (LV2) membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Displacement of [3H]-AVP from human vasopressin V2-receptor expressed in murine fibroblast cell (LV2) membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 1.44nMAssay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce...More data for this Ligand-Target Pair
Affinity DataIC50: 1.44nMpH: 8.0 T: 2°CAssay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMpH: 8.0 T: 2°CAssay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum...More data for this Ligand-Target Pair
Affinity DataIC50: 1.63nMAssay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce...More data for this Ligand-Target Pair
Affinity DataIC50: 1.63nMpH: 8.0 T: 2°CAssay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum...More data for this Ligand-Target Pair
Affinity DataIC50: 1.69nMpH: 8.0Assay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMpH: 8.0 T: 2°CAssay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce...More data for this Ligand-Target Pair
Affinity DataIC50: 1.79nMpH: 8.0 T: 2°CAssay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum...More data for this Ligand-Target Pair
Affinity DataIC50: 1.79nMAssay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce...More data for this Ligand-Target Pair
Affinity DataIC50: 1.84nMpH: 8.0 T: 2°CAssay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum...More data for this Ligand-Target Pair