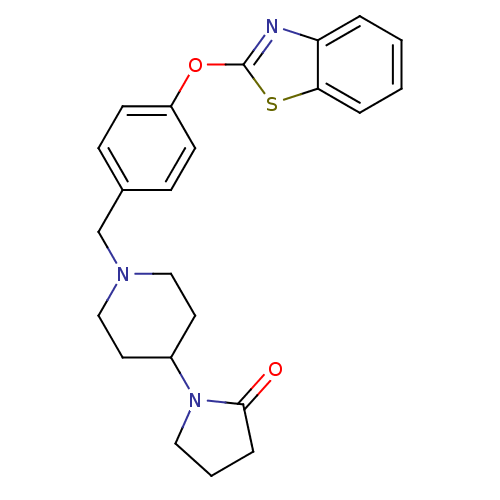
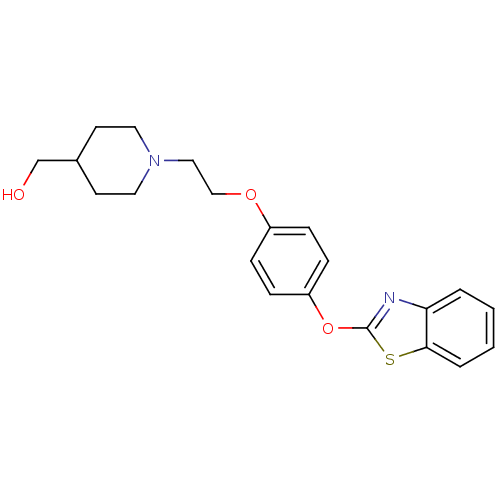
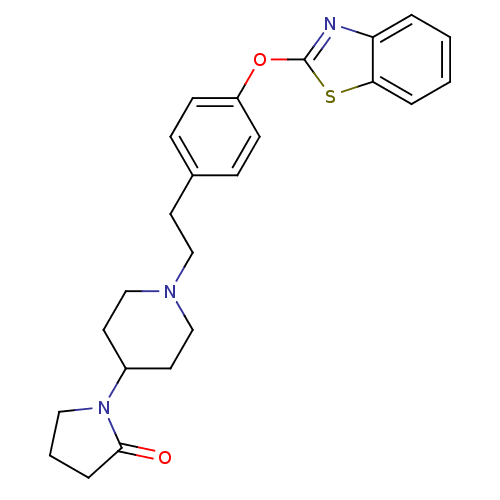
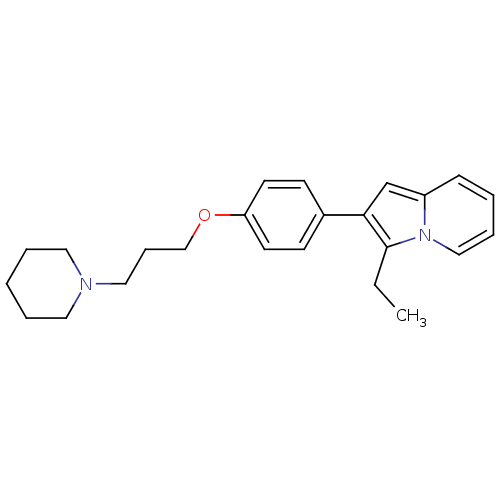
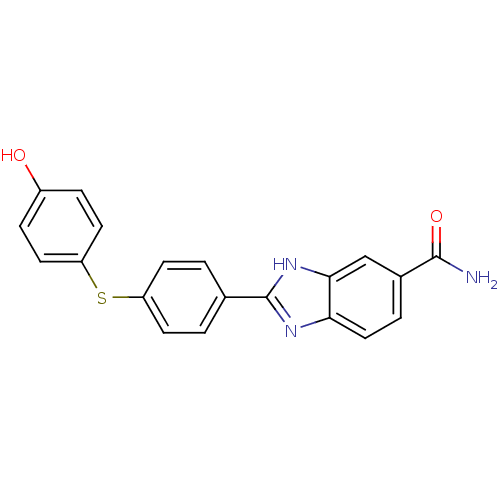
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligandMore data for this Ligand-Target Pair
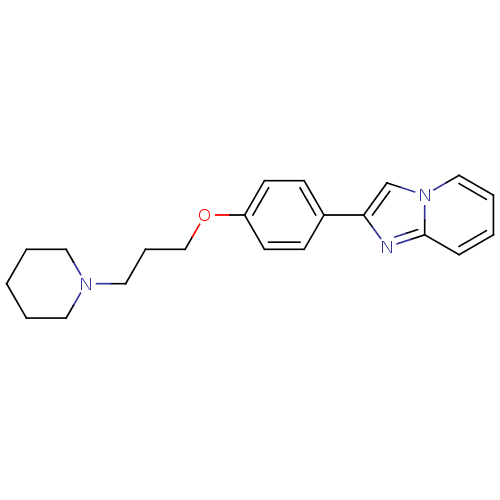
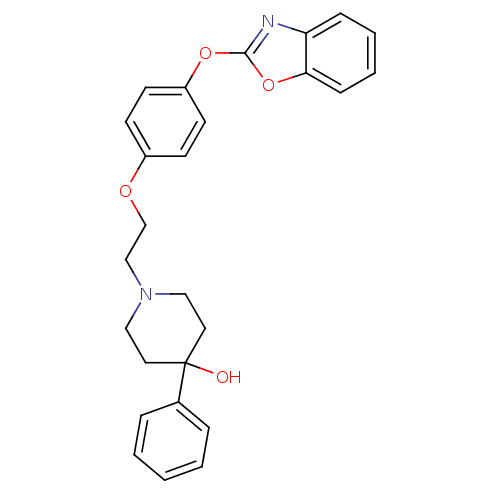
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligandMore data for this Ligand-Target Pair
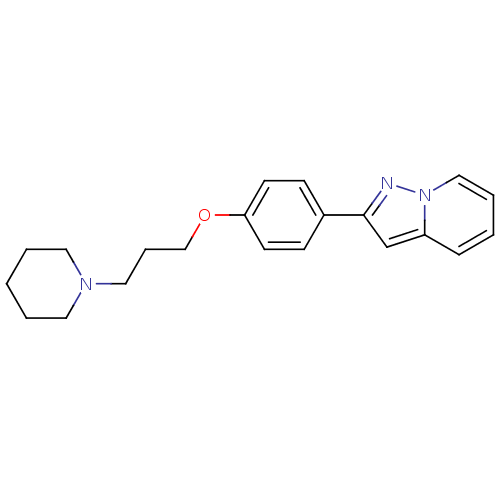
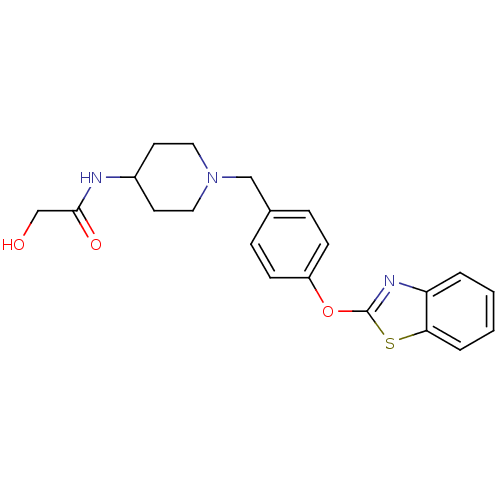
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 5nMAssay Description:Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligandMore data for this Ligand-Target Pair
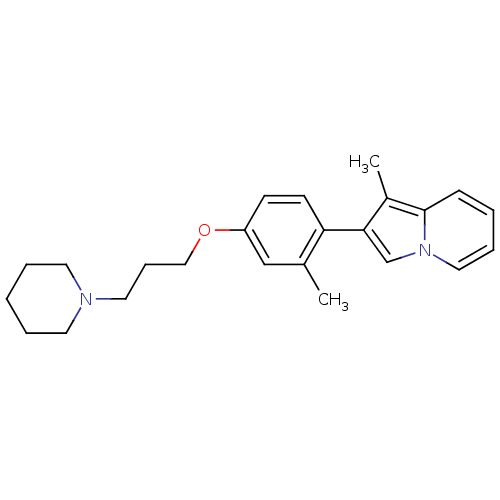
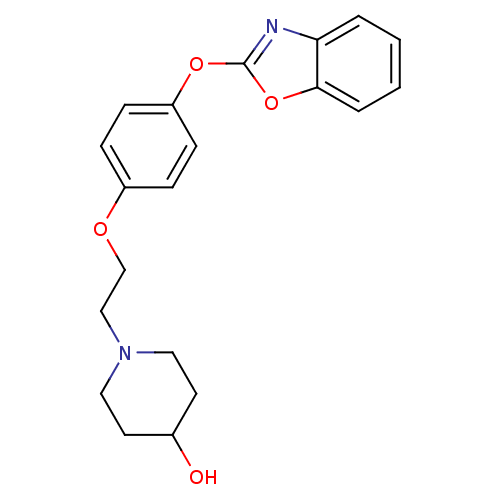
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 6nMAssay Description:Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligandMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligandMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligandMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 13nMAssay Description:Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligandMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 16nMAssay Description:Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligandMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 16nMAssay Description:Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligandMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 19nMAssay Description:Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligandMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 28nMAssay Description:Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligandMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 37nMAssay Description:Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligandMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 40nMAssay Description:Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligandMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 40nMAssay Description:Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligandMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 40nMAssay Description:Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligandMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 46nMAssay Description:Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligandMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 47nMAssay Description:Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligandMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 152nMAssay Description:Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligandMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 236nMAssay Description:Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligandMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 308nMAssay Description:Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligandMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of human recombinant Chk2More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 2.30nMAssay Description:Inhibition of recombinant human Chk2 kinaseMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 3.40nMAssay Description:Inhibition of recombinant human Chk2 kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 4.40nMAssay Description:Inhibition of recombinant human Chk2 kinaseMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 4.5nMAssay Description:Inhibition of recombinant human Chk2 kinaseMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 5.5nMAssay Description:Inhibition of recombinant human Chk2 kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 6.60nMAssay Description:Inhibition of recombinant human Chk2 kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 8nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 8nMT: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 8.20nMAssay Description:Inhibition of recombinant human Chk2 kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 9.80nMAssay Description:Inhibition of recombinant human Chk2 kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMT: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMT: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMT: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMT: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Inhibition of recombinant human Chk2 kinaseMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Inhibition of recombinant human Chk2 kinaseMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Inhibition of recombinant human Chk2 kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMT: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of recombinant human Chk2 kinaseMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 14nMAssay Description:Inhibition of human recombinant Chk2More data for this Ligand-Target Pair
Affinity DataIC50: 14nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair



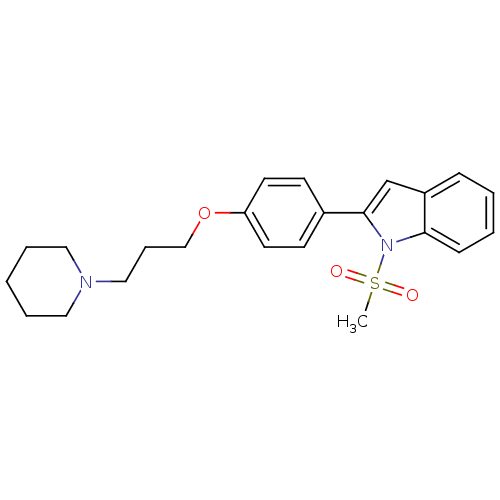
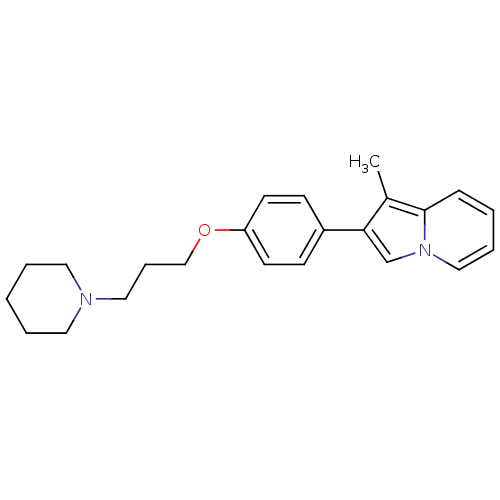


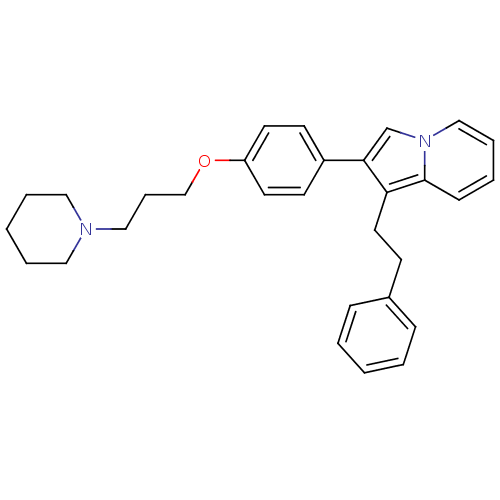

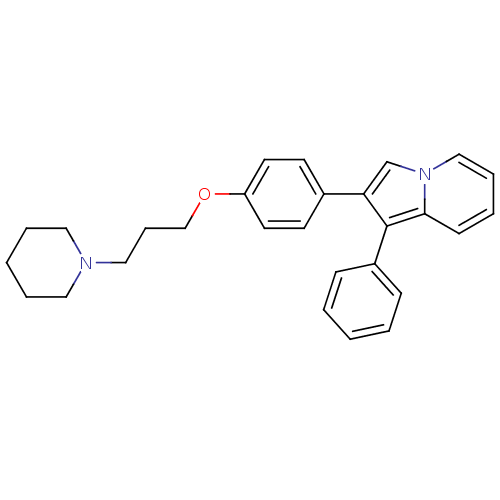
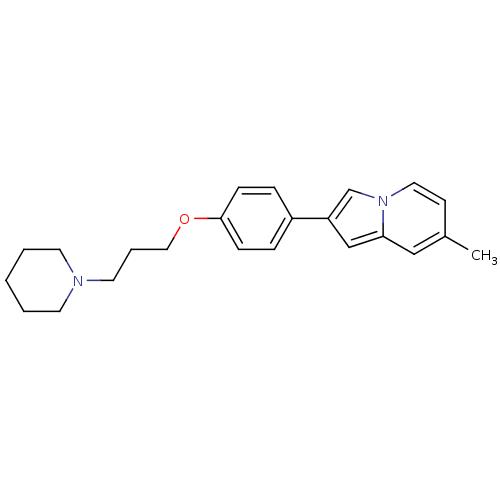
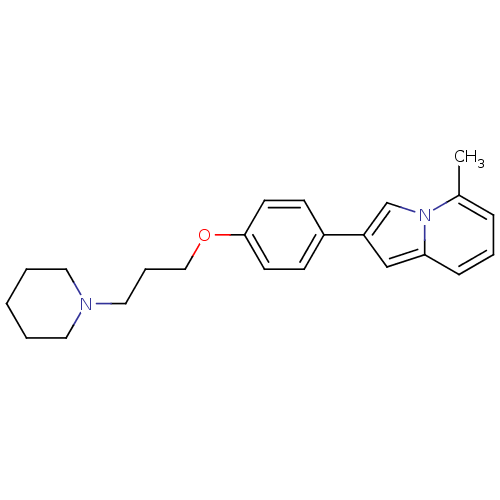
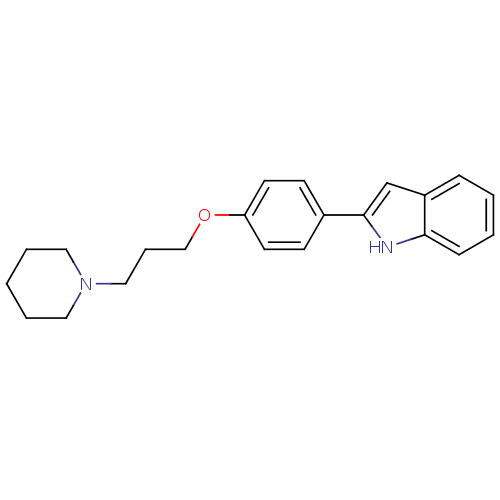

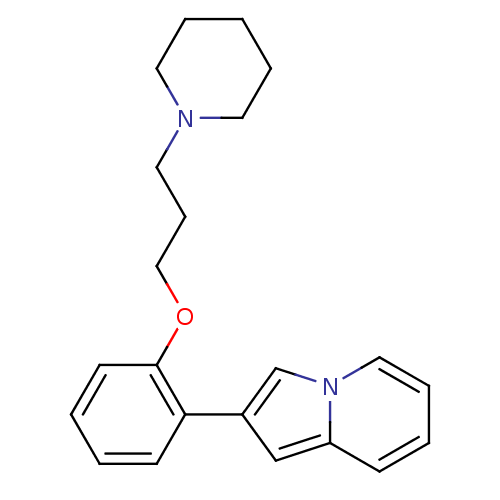











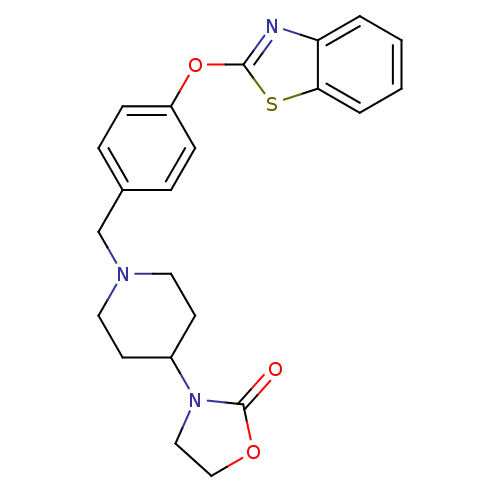
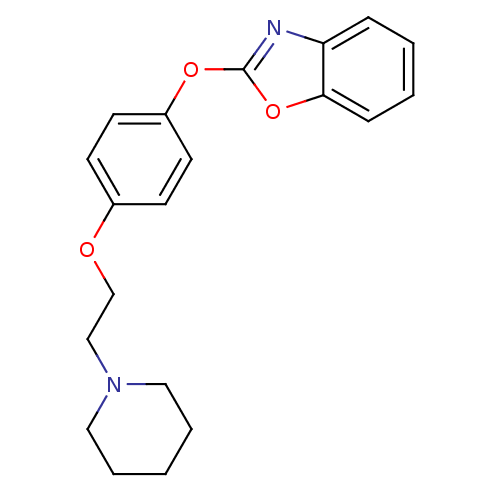
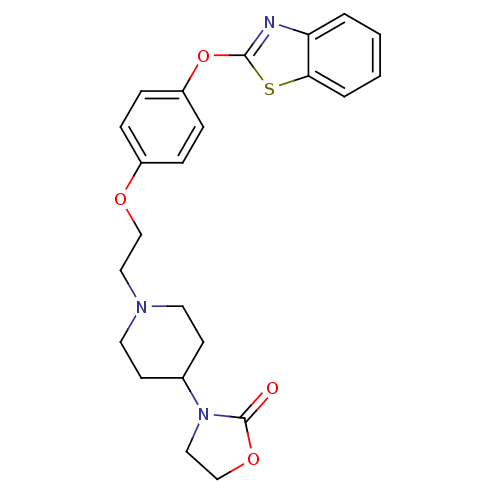
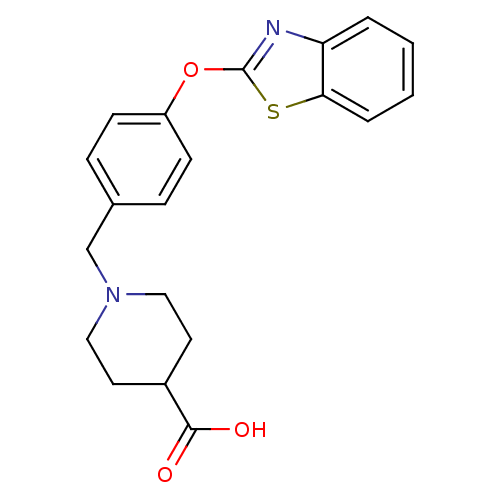
 3D Structure (crystal)
3D Structure (crystal)