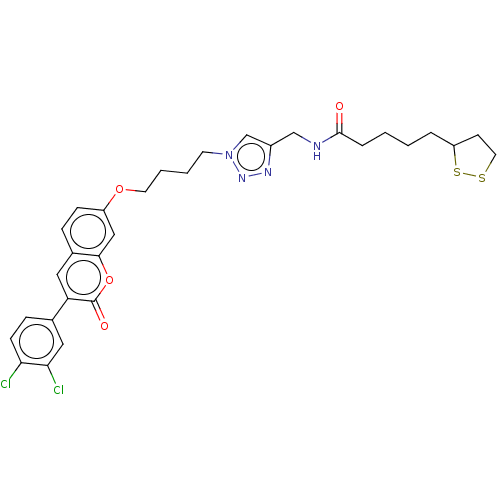
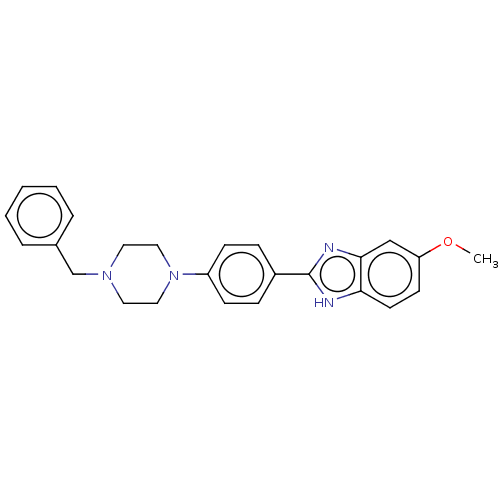
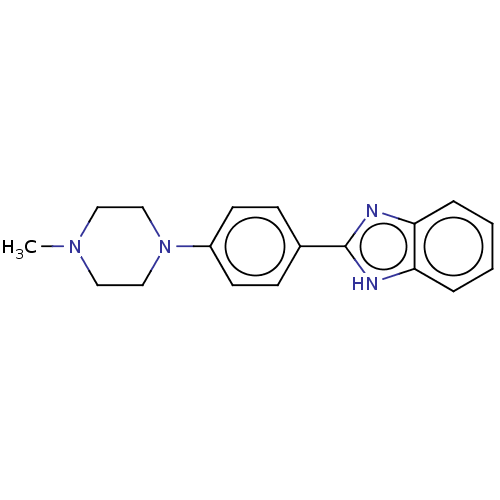
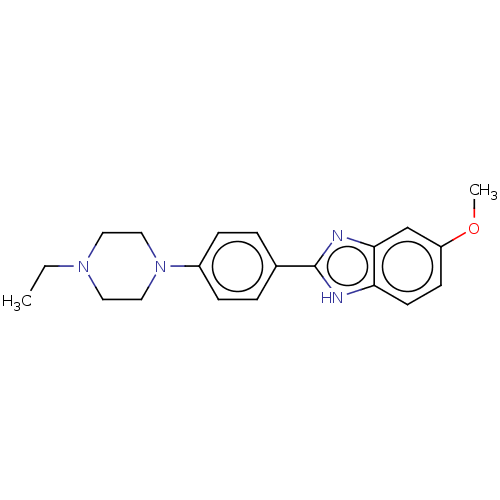
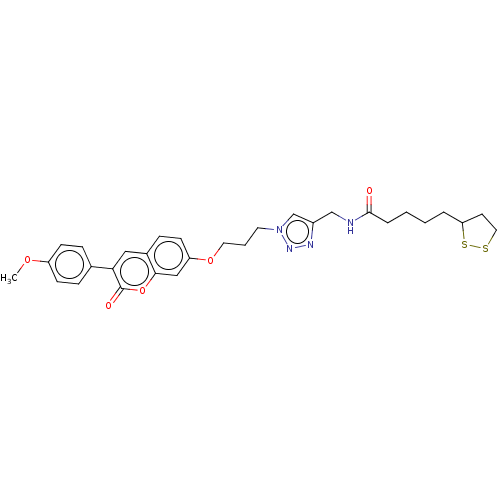
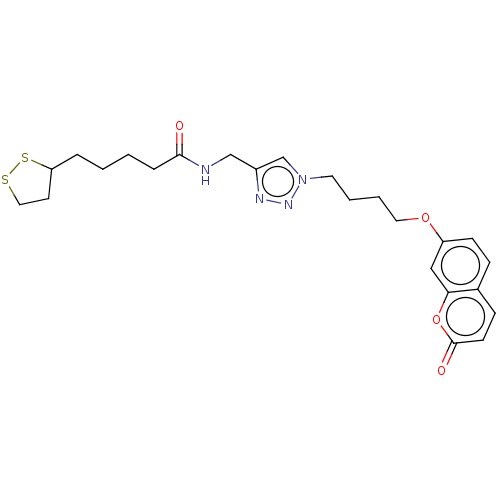
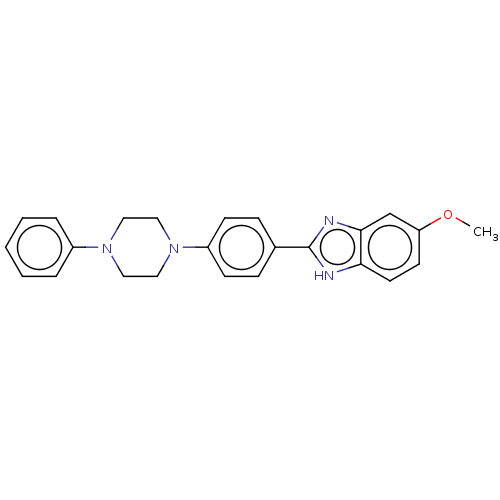
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Tehran University Of Medical Sciences
Curated by ChEMBL
Tehran University Of Medical Sciences
Curated by ChEMBL
Affinity DataKi: 6.91E+3nMAssay Description:Non-competitive inhibition of electric eel AChE using varying levels of acetylthiocholine iodide as substrate pretreated for 5 mins followed by subst...More data for this Ligand-Target Pair
Affinity DataIC50: 6.16nMpH: 8.0 T: 2°CAssay Description:Reactions were performed in a mediumcontaining substrate (0.05-0.4 mM) combined with 0.125 mM DTNB in 100 mM 3-(N-morpholino)propanesulfonic acid buf...More data for this Ligand-Target Pair
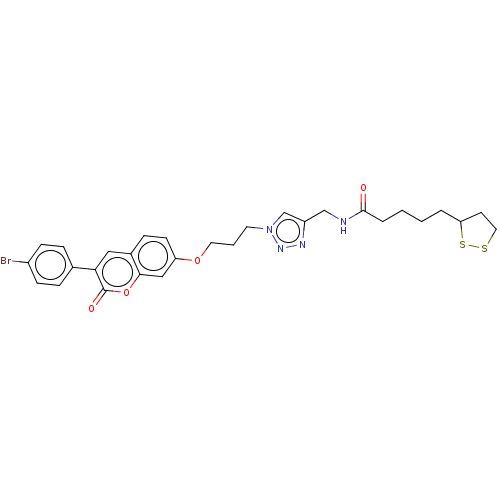
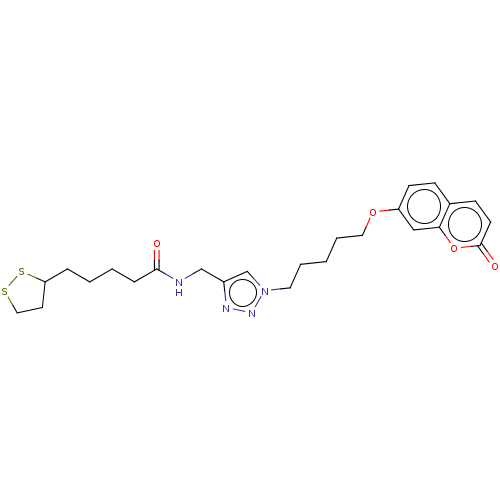
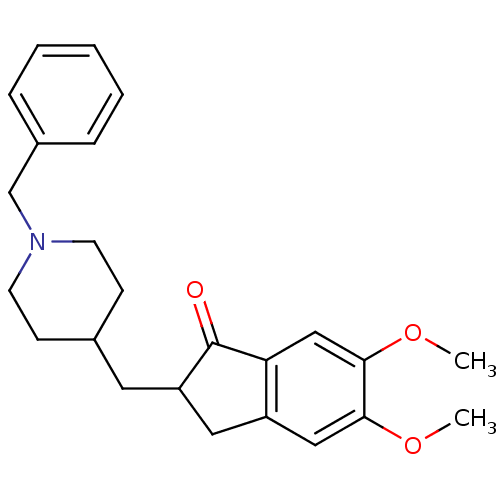
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Tehran University Of Medical Sciences
Curated by ChEMBL
Tehran University Of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.40E+3nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.94E+3nMpH: 8.0 T: 2°CAssay Description:Reactions were performed in a mediumcontaining substrate (0.05-0.4 mM) combined with 0.125 mM DTNB in 100 mM 3-(N-morpholino)propanesulfonic acid buf...More data for this Ligand-Target Pair
Affinity DataIC50: 5.18E+3nMpH: 8.0 T: 2°CAssay Description:Reactions were performed in a mediumcontaining substrate (0.05-0.4 mM) combined with 0.125 mM DTNB in 100 mM 3-(N-morpholino)propanesulfonic acid buf...More data for this Ligand-Target Pair
Affinity DataIC50: 5.22E+3nMpH: 8.0 T: 2°CAssay Description:Reactions were performed in a mediumcontaining substrate (0.05-0.4 mM) combined with 0.125 mM DTNB in 100 mM 3-(N-morpholino)propanesulfonic acid buf...More data for this Ligand-Target Pair
Affinity DataIC50: 7.80E+3nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.03E+4nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.15E+4nMpH: 8.0 T: 2°CAssay Description:Reactions were performed in a mediumcontaining substrate (0.05-0.4 mM) combined with 0.125 mM DTNB in 100 mM 3-(N-morpholino)propanesulfonic acid buf...More data for this Ligand-Target Pair
Affinity DataIC50: 1.57E+4nMpH: 8.0 T: 2°CAssay Description:Reactions were performed in a mediumcontaining substrate (0.05-0.4 mM) combined with 0.125 mM DTNB in 100 mM 3-(N-morpholino)propanesulfonic acid buf...More data for this Ligand-Target Pair
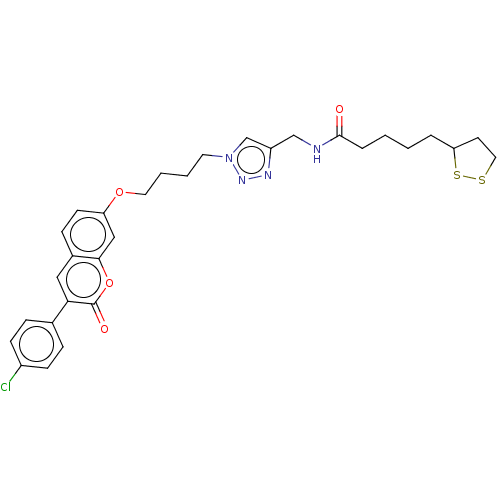
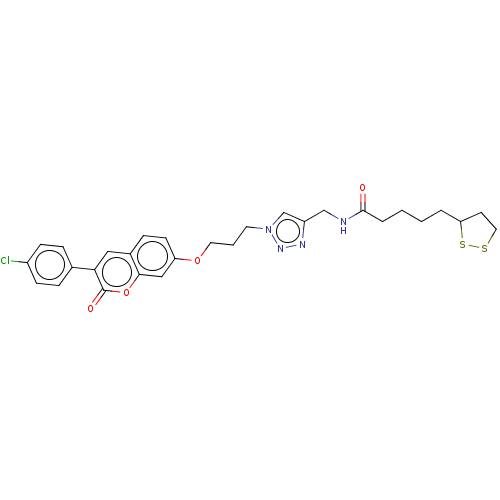
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Tehran University Of Medical Sciences
Curated by ChEMBL
Tehran University Of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 1.64E+4nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.78E+4nMpH: 8.0 T: 2°CAssay Description:Reactions were performed in a mediumcontaining substrate (0.05-0.4 mM) combined with 0.125 mM DTNB in 100 mM 3-(N-morpholino)propanesulfonic acid buf...More data for this Ligand-Target Pair
Affinity DataIC50: 2.02E+4nMpH: 8.0 T: 2°CAssay Description:Reactions were performed in a mediumcontaining substrate (0.05-0.4 mM) combined with 0.125 mM DTNB in 100 mM 3-(N-morpholino)propanesulfonic acid buf...More data for this Ligand-Target Pair
Affinity DataIC50: 3.49E+4nMpH: 8.0 T: 2°CAssay Description:Reactions were performed in a mediumcontaining substrate (0.05-0.4 mM) combined with 0.125 mM DTNB in 100 mM 3-(N-morpholino)propanesulfonic acid buf...More data for this Ligand-Target Pair
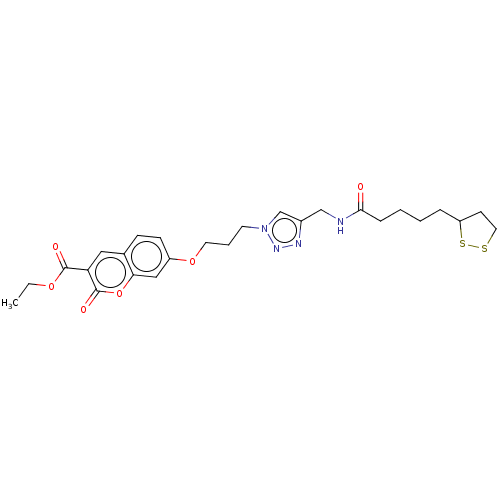
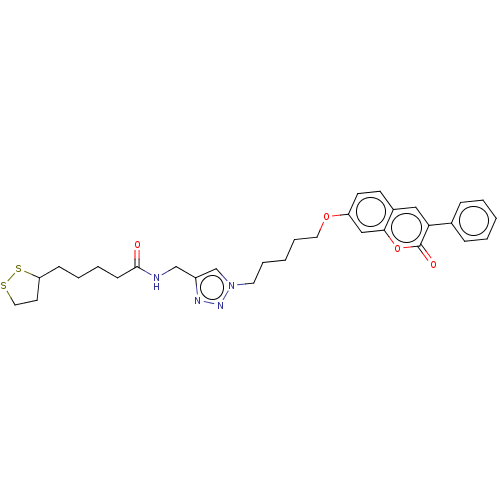
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Tehran University Of Medical Sciences
Curated by ChEMBL
Tehran University Of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 4.02E+4nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Tehran University Of Medical Sciences
Curated by ChEMBL
Tehran University Of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 4.65E+4nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.94E+4nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.20E+4nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Tehran University Of Medical Sciences
Curated by ChEMBL
Tehran University Of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 5.46E+4nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Tehran University Of Medical Sciences
Curated by ChEMBL
Tehran University Of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 5.50E+4nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Tehran University Of Medical Sciences
Curated by ChEMBL
Tehran University Of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 5.50E+4nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Tehran University Of Medical Sciences
Curated by ChEMBL
Tehran University Of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 5.97E+4nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Tehran University Of Medical Sciences
Curated by ChEMBL
Tehran University Of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 6.55E+4nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Tehran University Of Medical Sciences
Curated by ChEMBL
Tehran University Of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 6.67E+4nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Tehran University Of Medical Sciences
Curated by ChEMBL
Tehran University Of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 7.06E+4nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 7.35E+4nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 9.72E+4nMpH: 8.0 T: 2°CAssay Description:Reactions were performed in a mediumcontaining substrate (0.05-0.4 mM) combined with 0.125 mM DTNB in 100 mM 3-(N-morpholino)propanesulfonic acid buf...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Tehran University Of Medical Sciences
Curated by ChEMBL
Tehran University Of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Tehran University Of Medical Sciences
Curated by ChEMBL
Tehran University Of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Tehran University Of Medical Sciences
Curated by ChEMBL
Tehran University Of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Tehran University Of Medical Sciences
Curated by ChEMBL
Tehran University Of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Tehran University Of Medical Sciences
Curated by ChEMBL
Tehran University Of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Tehran University Of Medical Sciences
Curated by ChEMBL
Tehran University Of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMpH: 8.0 T: 2°CAssay Description:Reactions were performed in a mediumcontaining substrate (0.05-0.4 mM) combined with 0.125 mM DTNB in 100 mM 3-(N-morpholino)propanesulfonic acid buf...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMpH: 8.0 T: 2°CAssay Description:Reactions were performed in a mediumcontaining substrate (0.05-0.4 mM) combined with 0.125 mM DTNB in 100 mM 3-(N-morpholino)propanesulfonic acid buf...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMpH: 8.0 T: 2°CAssay Description:Reactions were performed in a mediumcontaining substrate (0.05-0.4 mM) combined with 0.125 mM DTNB in 100 mM 3-(N-morpholino)propanesulfonic acid buf...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMpH: 8.0 T: 2°CAssay Description:Reactions were performed in a mediumcontaining substrate (0.05-0.4 mM) combined with 0.125 mM DTNB in 100 mM 3-(N-morpholino)propanesulfonic acid buf...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)