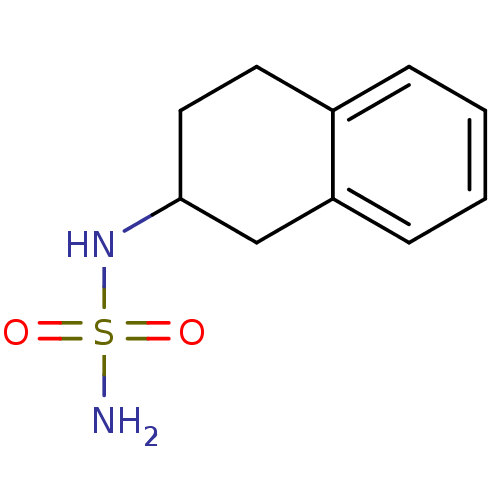
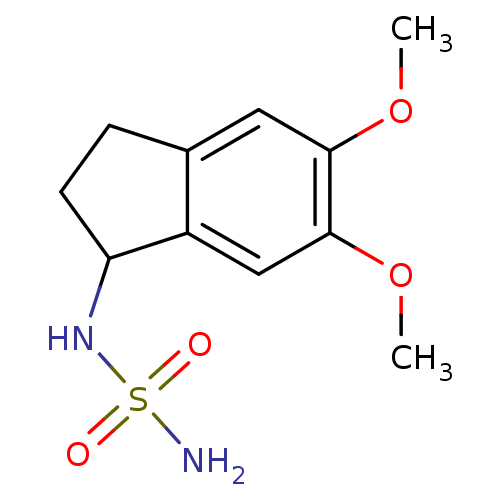
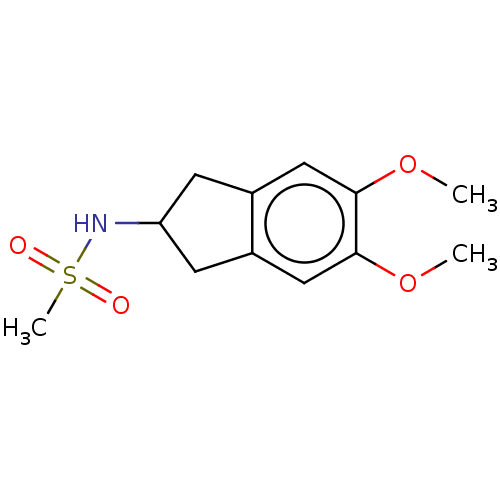
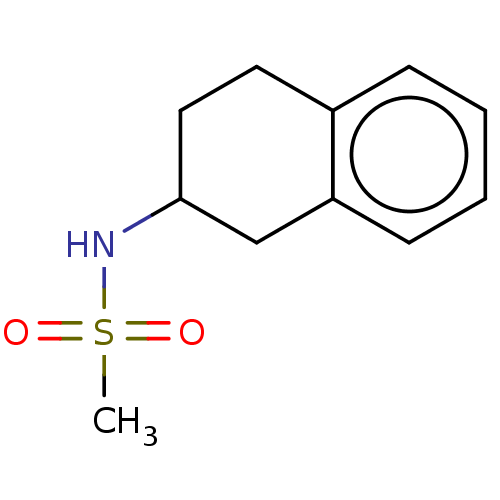
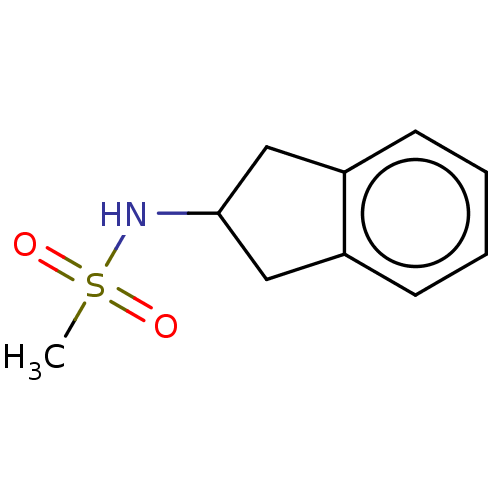
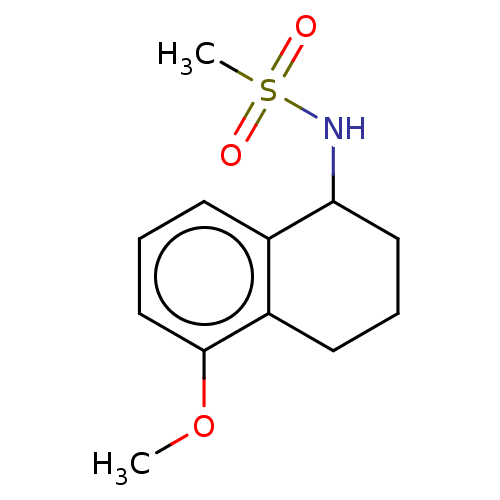
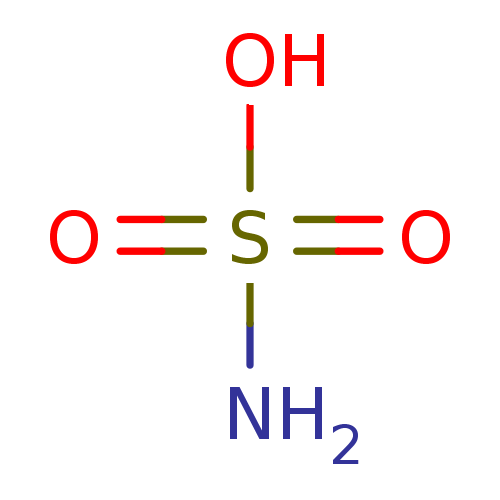
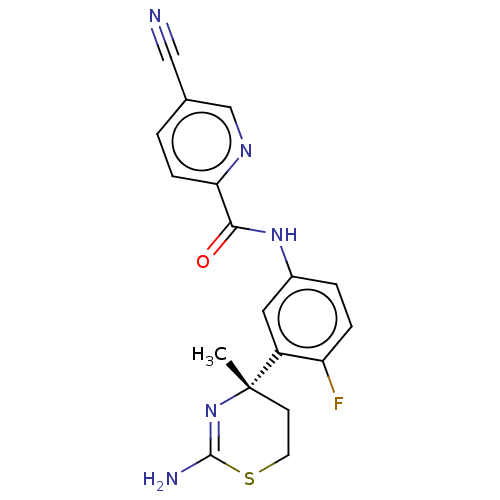
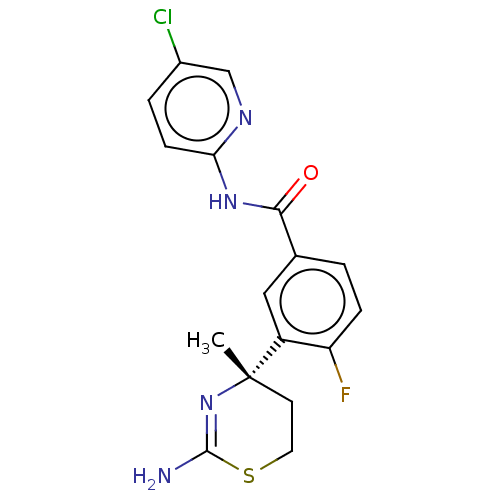
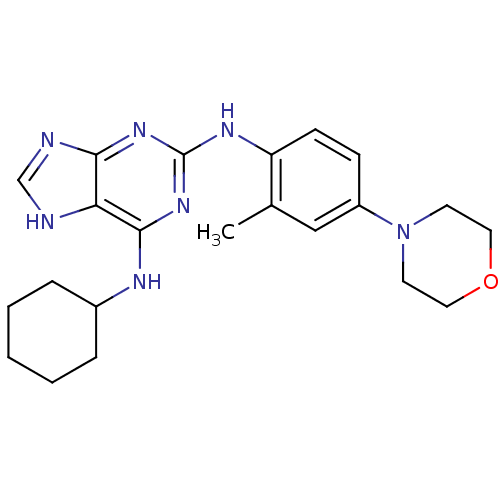
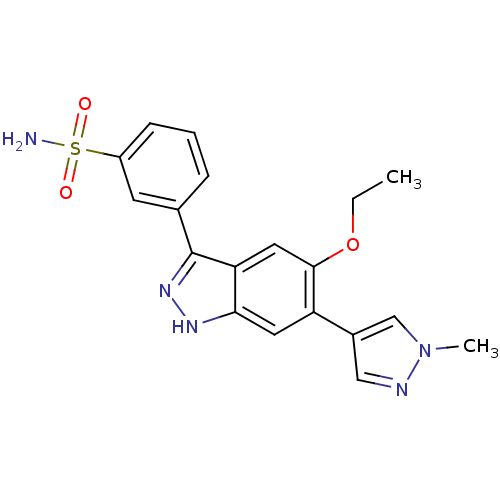
Affinity DataKi: 122nMAssay Description:Binding affinity for protein kinase C alphaMore data for this Ligand-Target Pair
Affinity DataKi: 122nMAssay Description:Binding affinity for protein kinase C alphaMore data for this Ligand-Target Pair
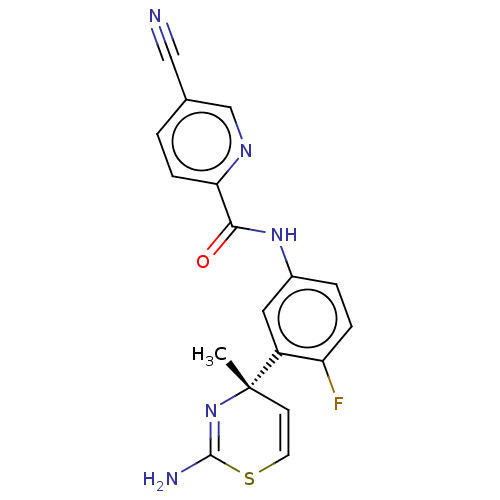
Affinity DataKi: 122nMAssay Description:Binding affinity for protein kinase C alphaMore data for this Ligand-Target Pair
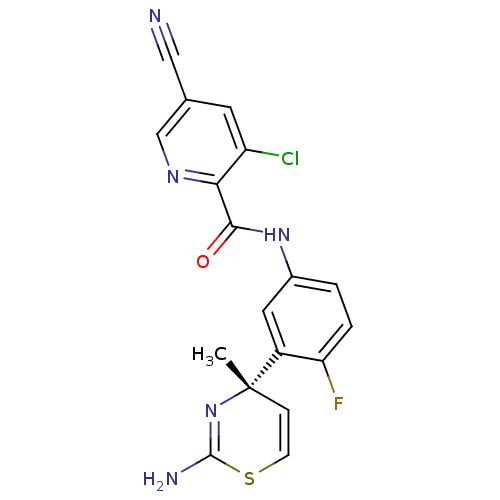
Affinity DataKi: 122nMAssay Description:Binding affinity for protein kinase C alphaMore data for this Ligand-Target Pair
Affinity DataKi: 450nMAssay Description:Inhibition of human erythrocytes esterase activity of carbonic anhydrase-1 using 4-nitrophenylacetate as substrate after 3 mins by Lineweaver-Burk pl...More data for this Ligand-Target Pair
Affinity DataKi: 540nMAssay Description:Binding affinity for protein kinase C alphaMore data for this Ligand-Target Pair
Affinity DataKi: 540nMAssay Description:Binding affinity for protein kinase C alphaMore data for this Ligand-Target Pair
Affinity DataKi: 540nMAssay Description:Binding affinity for protein kinase C alphaMore data for this Ligand-Target Pair
Affinity DataKi: 540nMAssay Description:Binding affinity for protein kinase C alphaMore data for this Ligand-Target Pair
Affinity DataKi: 1.07E+3nMAssay Description:Inhibition of human erythrocytes esterase activity of carbonic anhydrase-2 using 4-nitrophenylacetate as substrate after 3 mins by Lineweaver-Burk pl...More data for this Ligand-Target Pair
Affinity DataKi: 1.28E+3nMAssay Description:Inhibition of human erythrocytes esterase activity of carbonic anhydrase-2 using 4-nitrophenylacetate as substrate after 3 mins by Lineweaver-Burk pl...More data for this Ligand-Target Pair
Affinity DataKi: 1.54E+3nMAssay Description:Inhibition of human erythrocytes esterase activity of carbonic anhydrase-1 using 4-nitrophenylacetate as substrate after 3 mins by Lineweaver-Burk pl...More data for this Ligand-Target Pair
Affinity DataKi: 3.09E+3nMAssay Description:Inhibition of human erythrocytes esterase activity of carbonic anhydrase-2 using 4-nitrophenylacetate as substrate after 3 mins by Lineweaver-Burk pl...More data for this Ligand-Target Pair
Affinity DataKi: 3.70E+3nMAssay Description:Inhibition of human erythrocytes esterase activity of carbonic anhydrase-2 using 4-nitrophenylacetate as substrate after 3 mins by Lineweaver-Burk pl...More data for this Ligand-Target Pair
Affinity DataKi: 6.72E+3nMAssay Description:Inhibition of human erythrocytes esterase activity of carbonic anhydrase-1 using 4-nitrophenylacetate as substrate after 3 mins by Lineweaver-Burk pl...More data for this Ligand-Target Pair
Affinity DataKi: 6.75E+3nMAssay Description:Inhibition of human erythrocytes esterase activity of carbonic anhydrase-2 using 4-nitrophenylacetate as substrate after 3 mins by Lineweaver-Burk pl...More data for this Ligand-Target Pair
Affinity DataKi: 8.30E+3nMAssay Description:Inhibition of human erythrocytes esterase activity of carbonic anhydrase-1 using 4-nitrophenylacetate as substrate after 3 mins by Lineweaver-Burk pl...More data for this Ligand-Target Pair
Affinity DataKi: 8.86E+3nMAssay Description:Inhibition of human erythrocytes esterase activity of carbonic anhydrase-2 using 4-nitrophenylacetate as substrate after 3 mins by Lineweaver-Burk pl...More data for this Ligand-Target Pair
Affinity DataKi: 9.31E+3nMAssay Description:Inhibition of human erythrocytes esterase activity of carbonic anhydrase-1 using 4-nitrophenylacetate as substrate after 3 mins by Lineweaver-Burk pl...More data for this Ligand-Target Pair
Affinity DataKi: 1.02E+4nMAssay Description:Inhibition of human erythrocytes esterase activity of carbonic anhydrase-1 using 4-nitrophenylacetate as substrate after 3 mins by Lineweaver-Burk pl...More data for this Ligand-Target Pair
Affinity DataKi: 1.34E+4nMAssay Description:Inhibition of human erythrocytes esterase activity of carbonic anhydrase-2 using 4-nitrophenylacetate as substrate after 3 mins by Lineweaver-Burk pl...More data for this Ligand-Target Pair
Affinity DataKi: 3.62E+4nMAssay Description:Inhibition of human erythrocytes esterase activity of carbonic anhydrase-1 using 4-nitrophenylacetate as substrate after 3 mins by Lineweaver-Burk pl...More data for this Ligand-Target Pair
Affinity DataKi: 4.60E+4nM IC50: 2.66E+5nMAssay Description:The effect of novel sulfonamides 28-33 on HCA isozyme activity was determined colorimetrically using CO2-hydration method of Wilbur and Anderson29 de...More data for this Ligand-Target Pair
Affinity DataKi: 5.20E+4nM IC50: 4.87E+5nMAssay Description:The effect of novel sulfonamides 28-33 on HCA isozyme activity was determined colorimetrically using CO2-hydration method of Wilbur and Anderson29 de...More data for this Ligand-Target Pair
Affinity DataKi: 9.40E+4nM IC50: 3.38E+5nMAssay Description:The effect of novel sulfonamides 28-33 on HCA isozyme activity was determined colorimetrically using CO2-hydration method of Wilbur and Anderson29 de...More data for this Ligand-Target Pair
Affinity DataKi: 1.04E+5nMAssay Description:The effect of novel sulfonamides 28-33 on HCA isozyme activity was determined colorimetrically using CO2-hydration method of Wilbur and Anderson29 de...More data for this Ligand-Target Pair
Affinity DataKi: 1.12E+5nMAssay Description:The effect of novel sulfonamides 28-33 on HCA isozyme activity was determined colorimetrically using CO2-hydration method of Wilbur and Anderson29 de...More data for this Ligand-Target Pair
Affinity DataKi: 1.70E+5nMAssay Description:The effect of novel sulfonamides 28-33 on HCA isozyme activity was determined colorimetrically using CO2-hydration method of Wilbur and Anderson29 de...More data for this Ligand-Target Pair
Affinity DataKi: 1.73E+5nMAssay Description:The effect of novel sulfonamides 28-33 on HCA isozyme activity was determined colorimetrically using CO2-hydration method of Wilbur and Anderson29 de...More data for this Ligand-Target Pair
Affinity DataKi: 1.85E+5nMAssay Description:The effect of novel sulfonamides 28-33 on HCA isozyme activity was determined colorimetrically using CO2-hydration method of Wilbur and Anderson29 de...More data for this Ligand-Target Pair
Affinity DataKi: 2.11E+5nMAssay Description:The effect of novel sulfonamides 28-33 on HCA isozyme activity was determined colorimetrically using CO2-hydration method of Wilbur and Anderson29 de...More data for this Ligand-Target Pair
Affinity DataKi: 2.15E+5nMAssay Description:The effect of novel sulfonamides 28-33 on HCA isozyme activity was determined colorimetrically using CO2-hydration method of Wilbur and Anderson29 de...More data for this Ligand-Target Pair
Affinity DataKi: 2.28E+5nMAssay Description:The effect of novel sulfonamides 28-33 on HCA isozyme activity was determined colorimetrically using CO2-hydration method of Wilbur and Anderson29 de...More data for this Ligand-Target Pair
Affinity DataKi: 3.72E+5nMAssay Description:The effect of novel sulfonamides 28-33 on HCA isozyme activity was determined colorimetrically using CO2-hydration method of Wilbur and Anderson29 de...More data for this Ligand-Target Pair
Affinity DataKi: 3.90E+5nMAssay Description:Inhibition of human erythrocytes esterase activity of carbonic anhydrase-2 using 4-nitrophenylacetate as substrate after 3 mins by Lineweaver-Burk pl...More data for this Ligand-Target Pair
Affinity DataKi: 1.13E+6nMAssay Description:Inhibition of human erythrocytes esterase activity of carbonic anhydrase-2 using 4-nitrophenylacetate as substrate after 3 mins by Lineweaver-Burk pl...More data for this Ligand-Target Pair
TargetDual specificity protein kinase TTK(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of FLAG-tagged Mps1 autophosphorylation in human RERF-LC-AI cells expressing Tet-suppressible promotor after 3 hrs by immunoblottingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.620nMAssay Description:Inhibition of BACE1 in human SH-SY5Y cells expressing wild type betaAPP assessed as reduction in amyloidbeta (1 to 40 residues) level incubated for 2...More data for this Ligand-Target Pair
Affinity DataIC50: 0.630nMAssay Description:Inhibition of BACE1 in human SH-SY5Y cells expressing wild type betaAPP assessed as reduction in amyloidbeta (1 to 40 residues) level incubated for 2...More data for this Ligand-Target Pair
TargetDual specificity protein kinase TTK(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Inhibition of FLAG-tagged Mps1 autophosphorylation in human RERF-LC-AI cells expressing Tet-suppressible promotor after 3 hrs by immunoblottingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.840nMAssay Description:Inhibition of BACE1 in human SH-SY5Y cells expressing wild type betaAPP assessed as reduction in amyloidbeta (1 to 40 residues) level incubated for 2...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Inhibition of BACE1 in human SH-SY5Y cells expressing wild type betaAPP assessed as reduction in amyloidbeta (1 to 40 residues) level incubated for 2...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Inhibition of BACE1 in human SH-SY5Y cells expressing wild type betaAPP assessed as reduction in amyloidbeta (1 to 40 residues) level incubated for 2...More data for this Ligand-Target Pair
TargetDual specificity protein kinase TTK(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 1.30nMAssay Description:Inhibition of FLAG-tagged Mps1 autophosphorylation in human RERF-LC-AI cells expressing Tet-suppressible promotor after 3 hrs by immunoblottingMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Inhibition of BACE1 in human SH-SY5Y cells expressing wild type betaAPP assessed as reduction in amyloidbeta (1 to 40 residues) level incubated for 2...More data for this Ligand-Target Pair
TargetDual specificity protein kinase TTK(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of human MPS1 expressed in Escherichia coliMore data for this Ligand-Target Pair
TargetDual specificity protein kinase TTK(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 2.5nMAssay Description:Inhibition of Mps1 (unknown origin)-mediated p38 MAPK phosphorylation after 90 mins by DELFIA assayMore data for this Ligand-Target Pair
TargetDual specificity protein kinase TTK(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 2.60nMAssay Description:Inhibition of Mps1 (unknown origin)-mediated p38 MAPK phosphorylation after 90 mins by DELFIA assayMore data for this Ligand-Target Pair
TargetDual specificity protein kinase TTK(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 2.80nMAssay Description:Inhibition of Mps1 (unknown origin)-mediated p38 MAPK phosphorylation after 90 mins by DELFIA assayMore data for this Ligand-Target Pair
TargetDual specificity protein kinase TTK(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 3.10nMAssay Description:Inhibition of MPS1 (unknown origin) using biotin-labeled AGAGLARHTDDEMTGYVA as substrate after 90 mins by DELFIAMore data for this Ligand-Target Pair

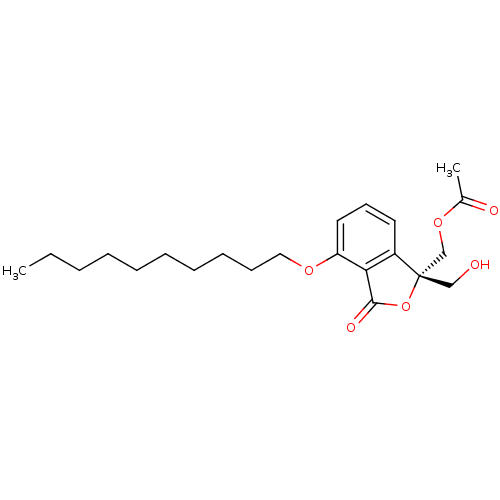
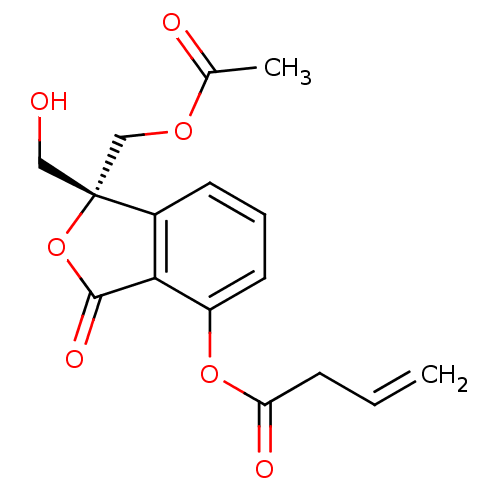

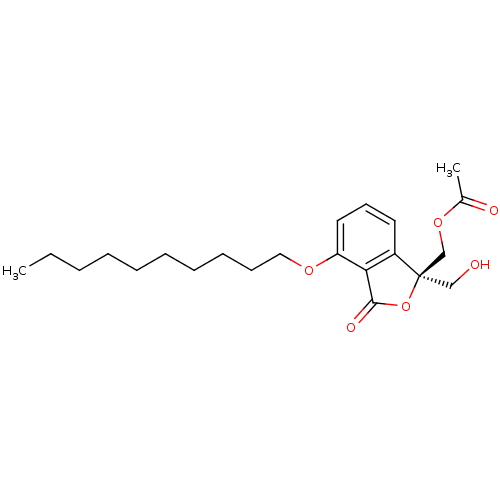


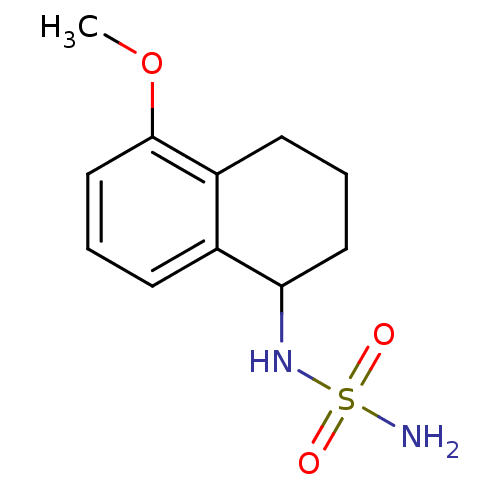
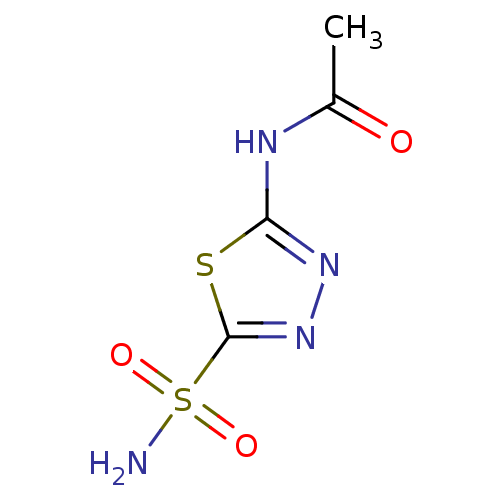
 3D Structure (crystal)
3D Structure (crystal)