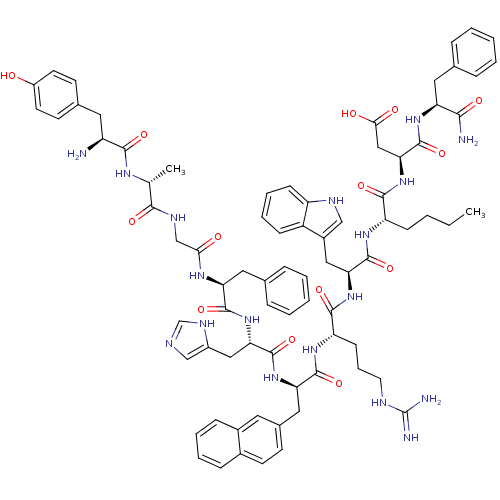
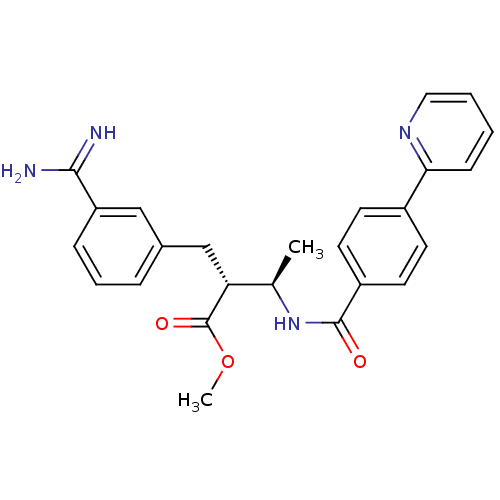
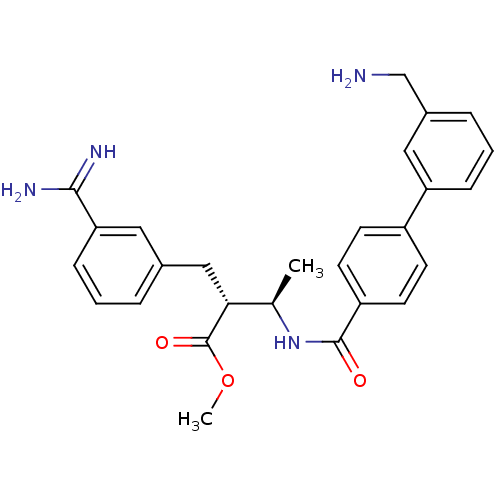
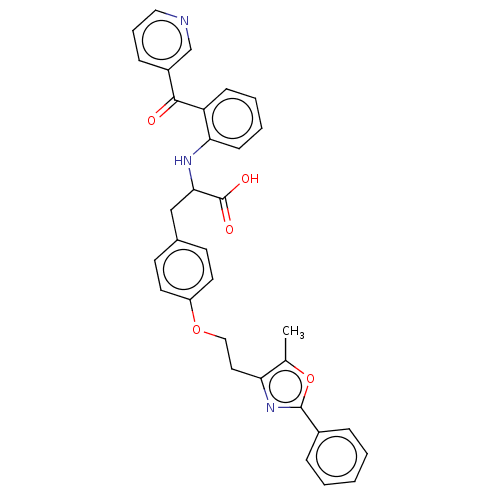
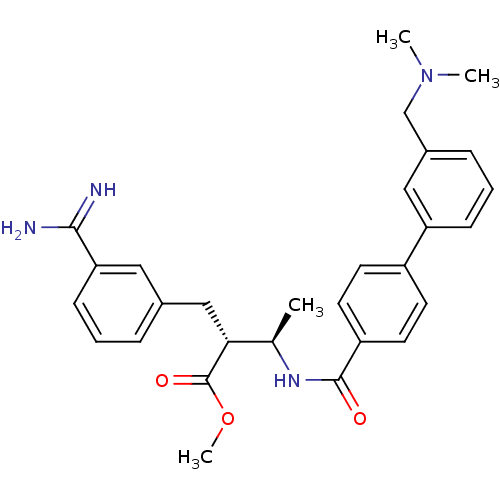
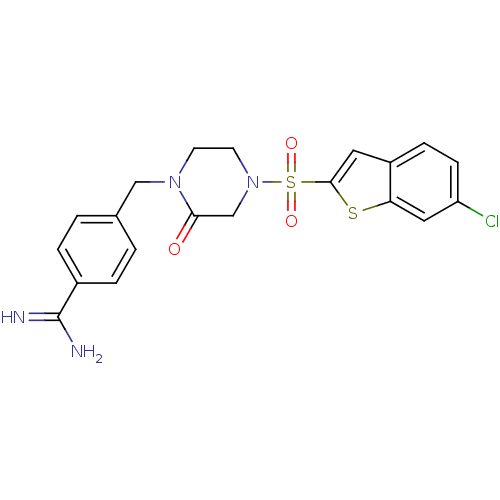
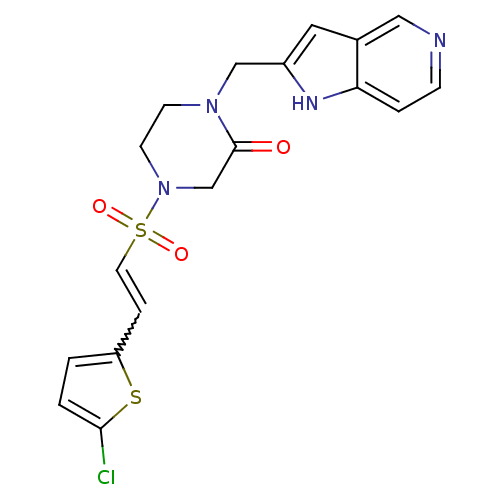
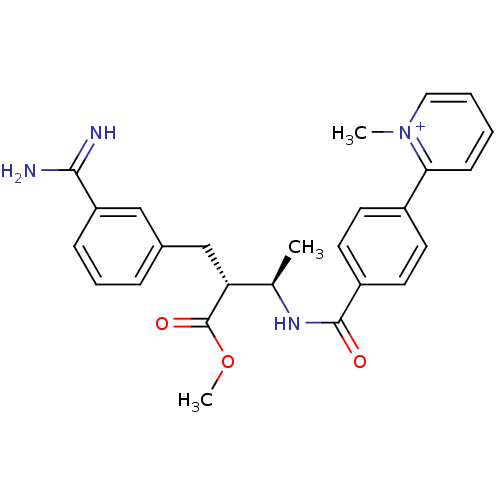
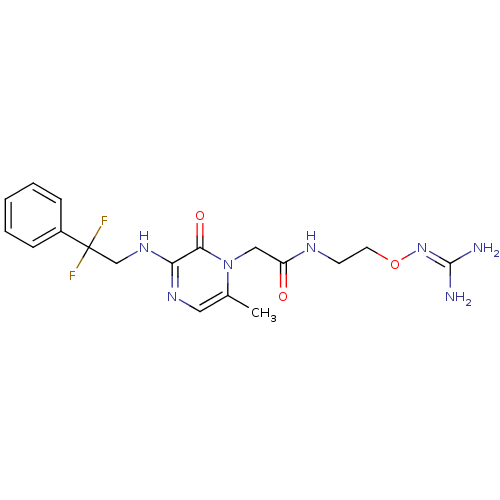
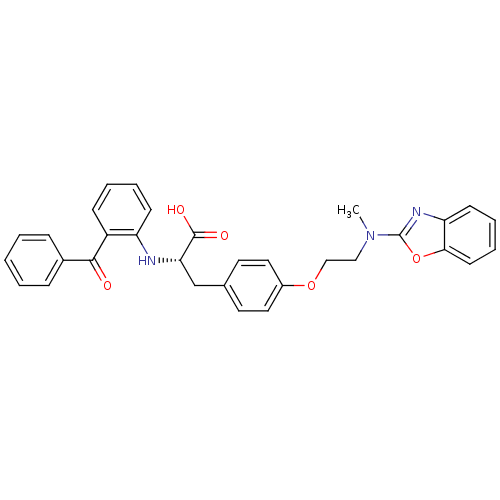
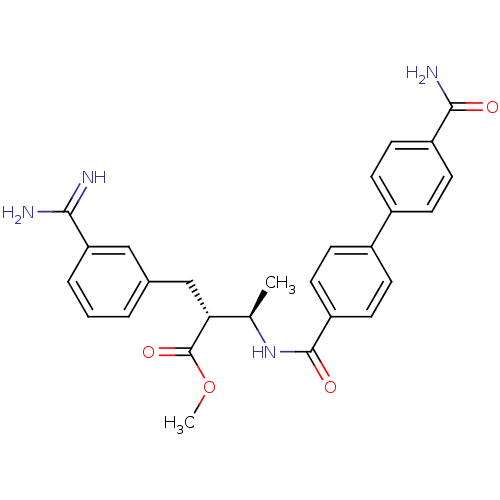
Affinity DataKi: 0.0470nMAssay Description:Compound was evaluated for the inhibition of human Coagulation factor XaMore data for this Ligand-Target Pair
TargetP2Y purinoceptor 14(Homo sapiens (Human))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
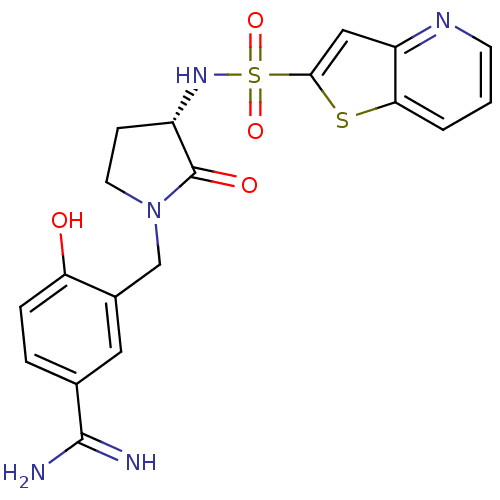
Affinity DataKi: 0.0800nMAssay Description:Antagonist activity at human P2Y14R expressed in CHO cells assessed as inhibition of UDPG-mediated reduction of forskolin-induced [3H]cAMP production...More data for this Ligand-Target Pair
TargetP2Y purinoceptor 14(Homo sapiens (Human))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
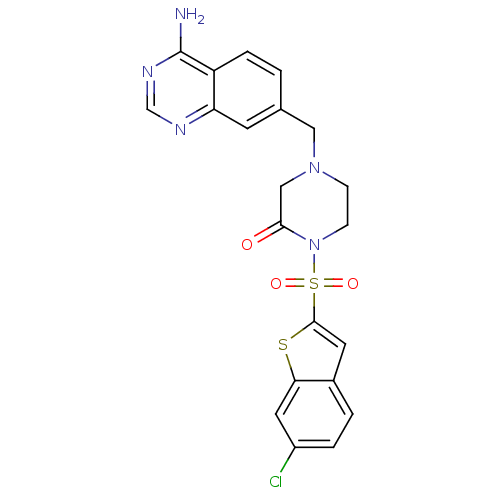
Affinity DataKi: 0.0800nMAssay Description:Antagonist activity at human P2Y14R expressed in CHO cells assessed as inhibition of UDPG-mediated reduction of forskolin-induced [3H]cAMP production...More data for this Ligand-Target Pair
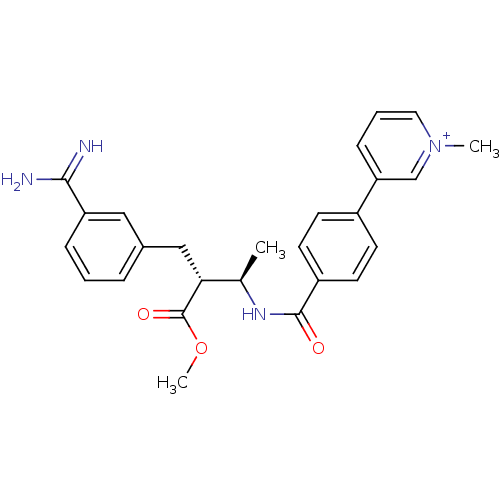
Affinity DataKi: 0.130nMAssay Description:Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.130nMAssay Description:Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.180nMAssay Description:Inhibition of [3H]nociceptin binding to human Opioid receptor like 1More data for this Ligand-Target Pair
Affinity DataKi: 0.380nMAssay Description:Competitive inhibition of human Aurora B ATP binding site by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataKi: 0.380nMAssay Description:Competitive inhibition of Aurora B ATP binding siteMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:In vitro inhibitory activity against human Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:In vitro inhibition of coagulation factor Xa.More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:In vitro inhibitory activity against human Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 0.580nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Inhibition of Aurora AMore data for this Ligand-Target Pair
Affinity DataKi: 0.690nMAssay Description:Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:In vitro inhibitory activity against human Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:Binding affinity against serine protease factor Xa (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 0.75nMAssay Description:In vitro inhibition of coagulation factor Xa.More data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:Binding affinity (in vitro) of the compound towards human Coagulation factor X was determined at 10 mg/kg peroral doseMore data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 0.890nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 0.900nMAssay Description:In vitro inhibitory activity against human Coagulation factor XaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Glaxo Wellcome Research And Development
Curated by ChEMBL
Glaxo Wellcome Research And Development
Curated by ChEMBL
Affinity DataKi: 0.933nMAssay Description:Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma)More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Glaxo Wellcome Research And Development
Curated by ChEMBL
Glaxo Wellcome Research And Development
Curated by ChEMBL
Affinity DataKi: 0.977nMAssay Description:Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma)More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:In vitro inhibitory activity against human Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Inhibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Inhibition of Coagulation factor XaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Glaxo Wellcome Research And Development
Curated by ChEMBL
Glaxo Wellcome Research And Development
Curated by ChEMBL
Affinity DataKi: 1.10nMAssay Description:Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma)More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Glaxo Wellcome Research And Development
Curated by ChEMBL
Glaxo Wellcome Research And Development
Curated by ChEMBL
Affinity DataKi: 1.10nMAssay Description:In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa...Checked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Binding affinity (in vitro) of the compound towards human Coagulation factor X was determined at 5 mg/kg peroral doseMore data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.30nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:Compound was evaluated for the inhibitory activity against Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Binding affinity against serine protease factor Xa (fXa)More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Glaxo Wellcome Research And Development
Curated by ChEMBL
Glaxo Wellcome Research And Development
Curated by ChEMBL
Affinity DataKi: 1.5nMAssay Description:Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma)More data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Competitive inhibition of human Aurora C ATP binding siteMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Glaxo Wellcome Research And Development
Curated by ChEMBL
Glaxo Wellcome Research And Development
Curated by ChEMBL
Affinity DataKi: 1.5nMAssay Description:In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa...Checked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:Displacement of [125I]CCK-8(SO3) from human CCK1 receptor expressed in human HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:Displacement of [125I]CCK-8(SO3) from human CCK1 receptor expressed in human HEK293 cellsMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Glaxo Wellcome Research And Development
Curated by ChEMBL
Glaxo Wellcome Research And Development
Curated by ChEMBL
Affinity DataKi: 1.60nMAssay Description:Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma)More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Glaxo Wellcome Research And Development
Curated by ChEMBL
Glaxo Wellcome Research And Development
Curated by ChEMBL
Affinity DataKi: 1.70nMAssay Description:Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma)More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.70nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:Competitive inhibition of Aurora B ATP binding siteMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Glaxo Wellcome Research And Development
Curated by ChEMBL
Glaxo Wellcome Research And Development
Curated by ChEMBL
Affinity DataKi: 1.80nMAssay Description:Binding affinity against peroxisome proliferator activated receptor gamma (PPAR-gamma)More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Binding affinity against serine protease Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:In vitro inhibitory activity against human Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:In vitro inhibition of coagulation factor Xa.More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Binding affinity against serine protease factor Xa (fXa)More data for this Ligand-Target Pair

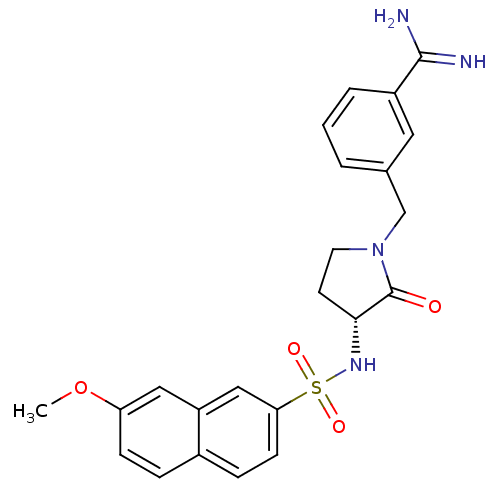
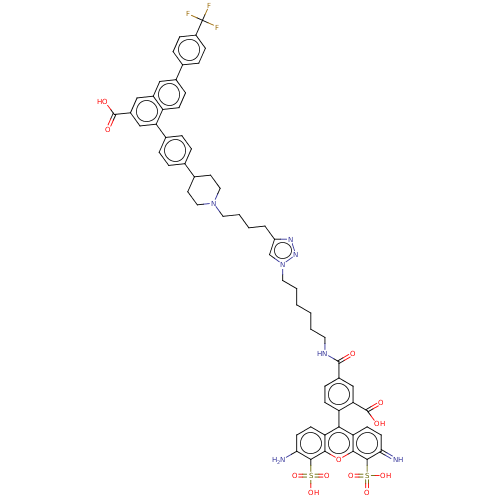
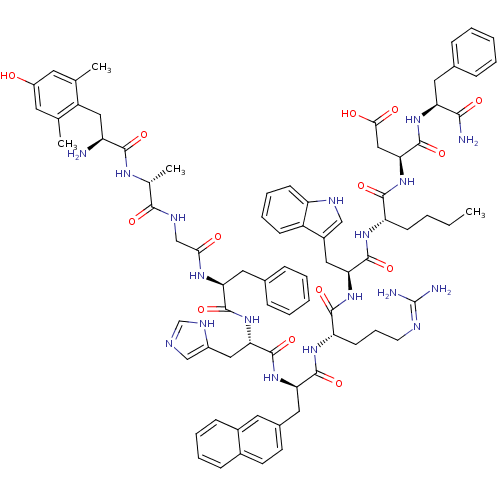
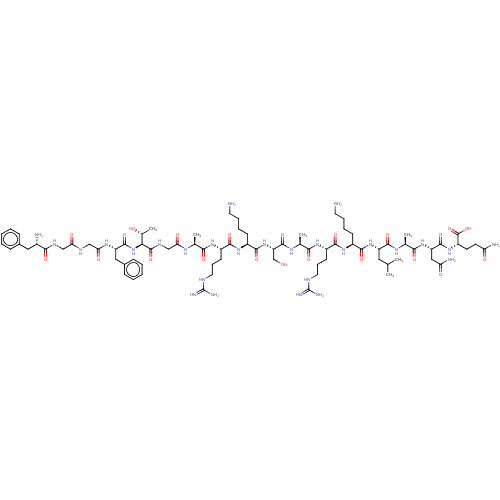
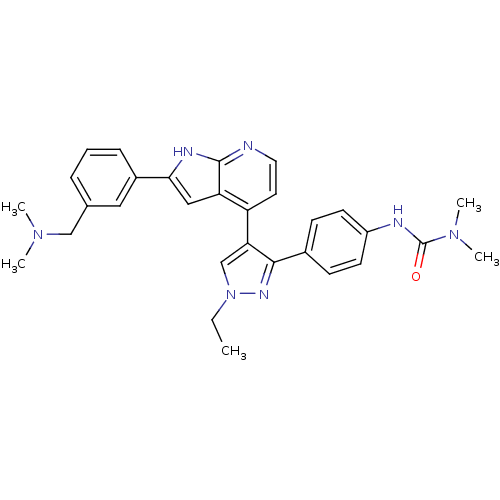
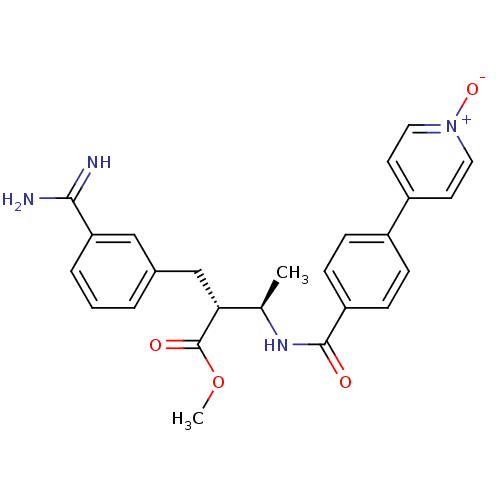

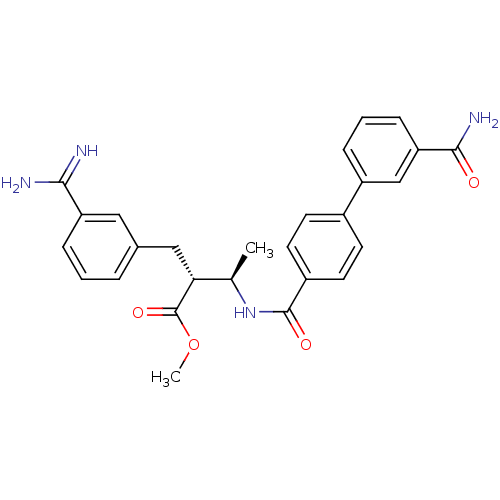
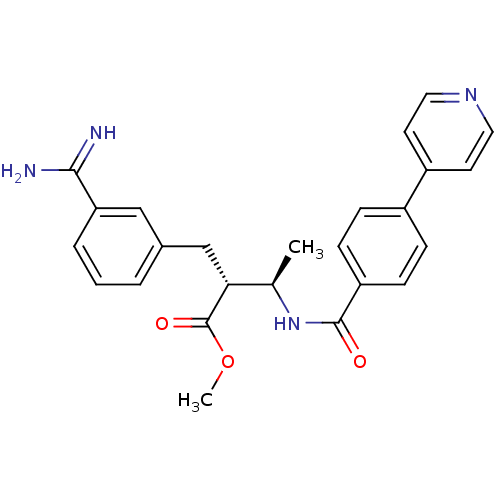
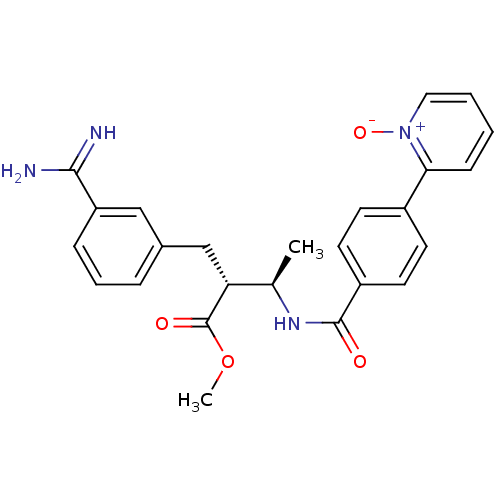
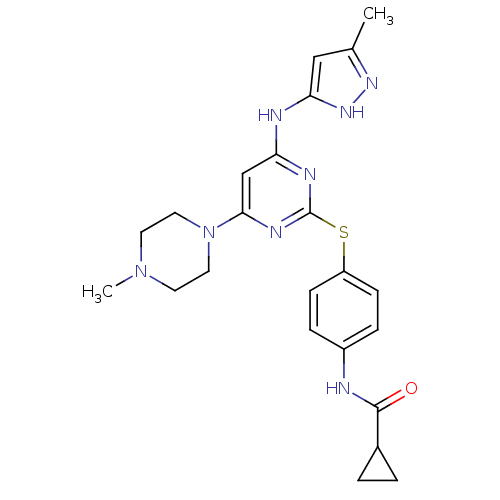
 3D Structure (crystal)
3D Structure (crystal)