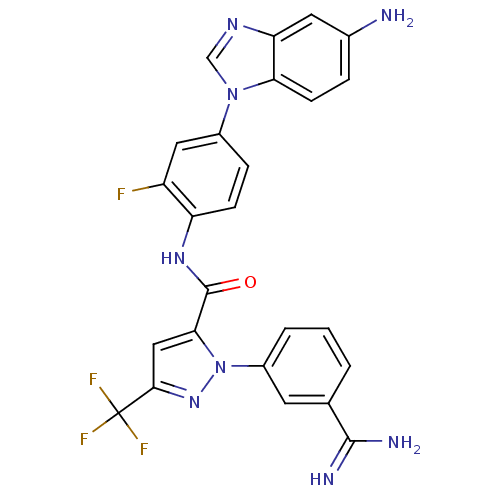
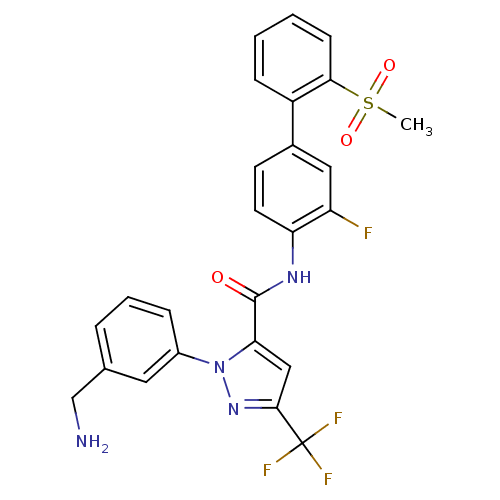
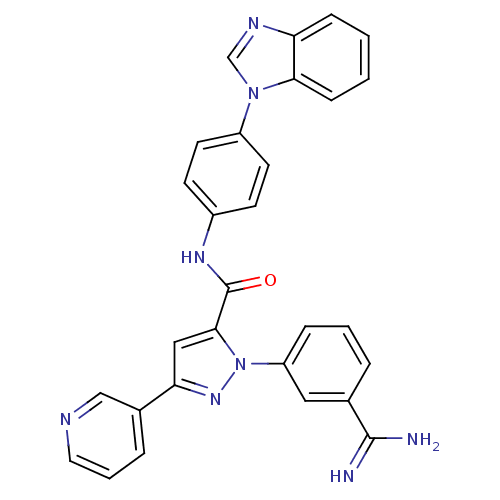
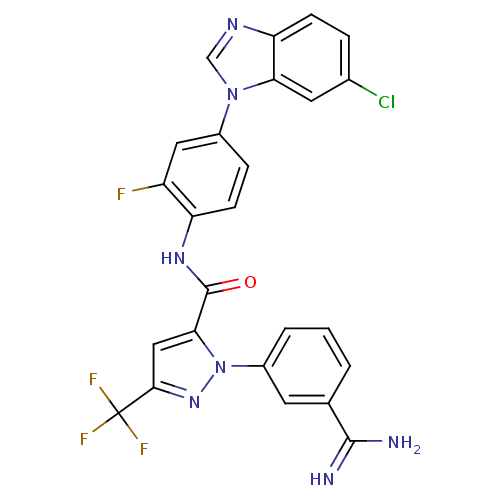
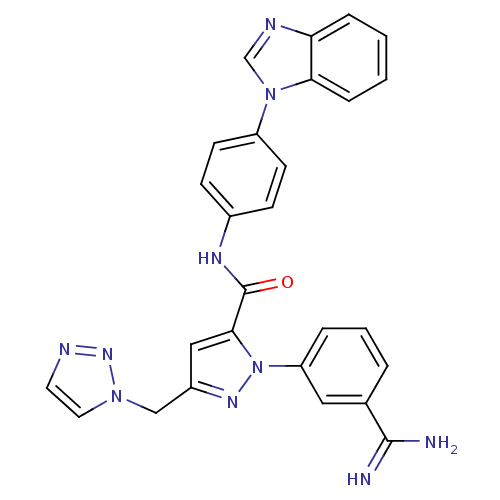
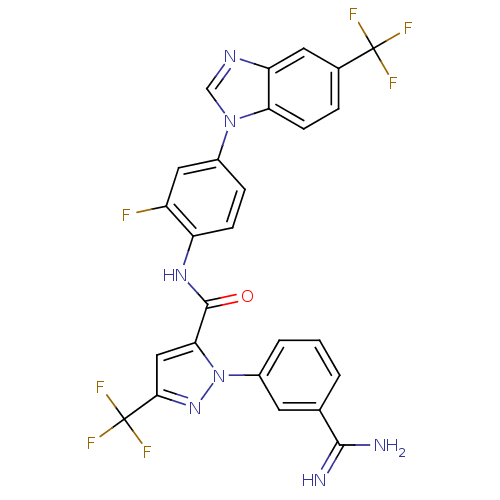
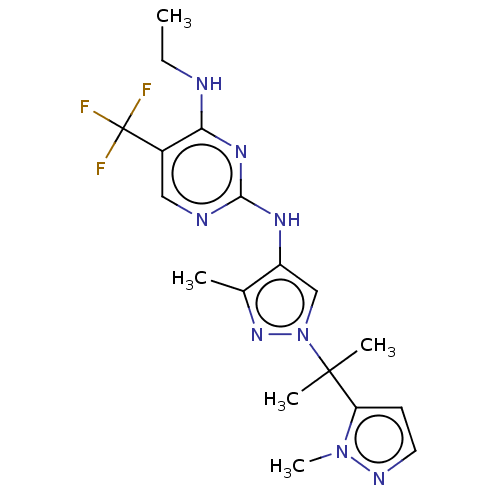
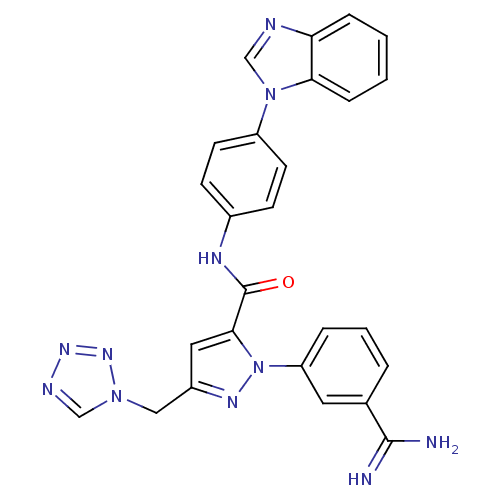
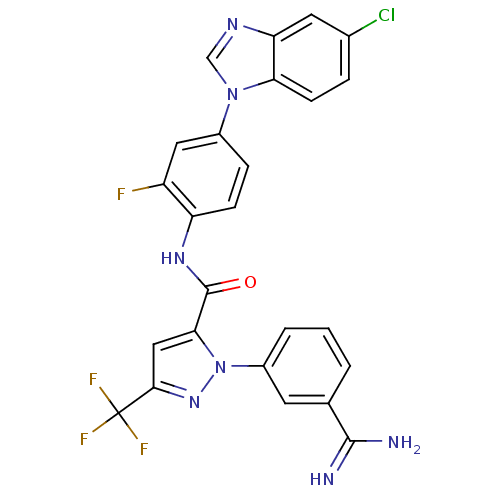
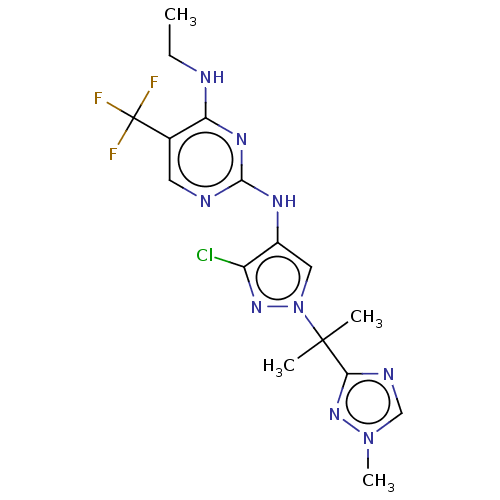
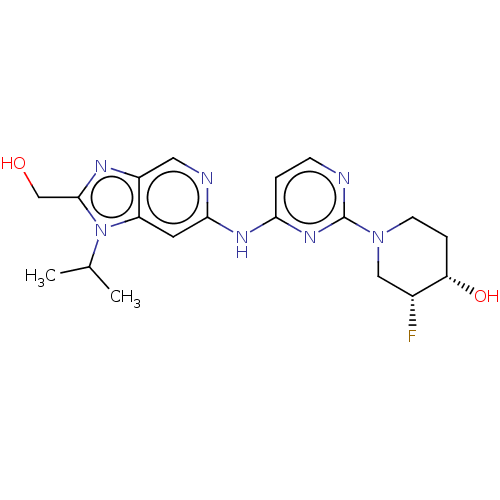
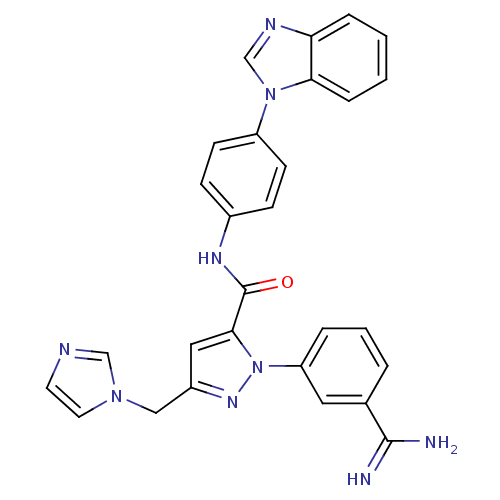
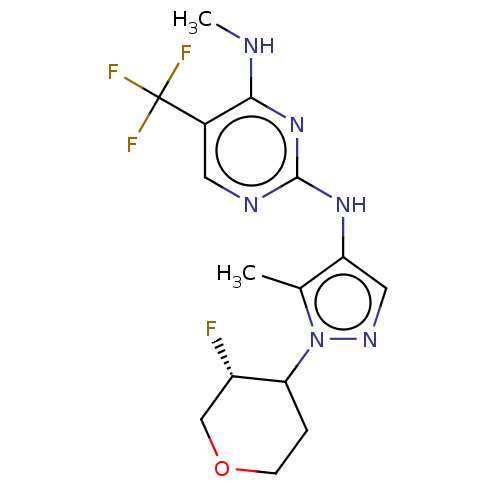
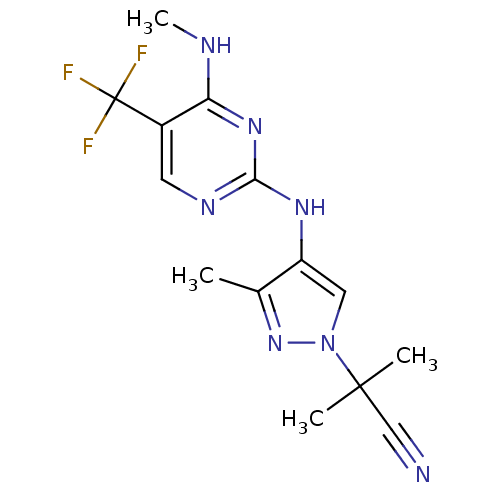
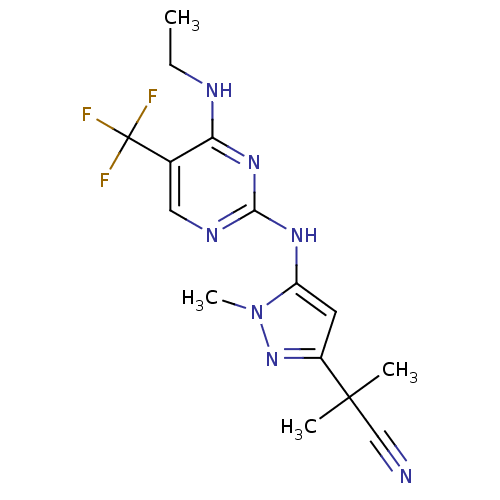
Affinity DataKi: 0.0130nM ΔG°: -61.5kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 0.0130nMAssay Description:Tested in vitro for inhibition of human Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 0.0240nM ΔG°: -60.0kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 0.0280nM ΔG°: -59.6kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 0.0360nM ΔG°: -59.0kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 0.0440nM ΔG°: -58.5kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 0.0580nM ΔG°: -57.8kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 0.0800nM ΔG°: -57.1kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 0.0910nM ΔG°: -56.7kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 0.0940nM ΔG°: -56.7kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 0.100nM ΔG°: -56.5kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 0.100nMAssay Description:Inhibition of EGFR T790M/L858R mutant (unknown origin) by high-throughput biochemical screeningMore data for this Ligand-Target Pair
Affinity DataKi: 0.100nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged EGFR catalytic domain (669 to 1210 residues) expressed in baculovirus expression system by mass...More data for this Ligand-Target Pair
Affinity DataKi: 0.100nMAssay Description:Inhibition of wild-type EGFR (unknown origin) by high-throughput biochemical screeningMore data for this Ligand-Target Pair
Affinity DataKi: 0.120nM ΔG°: -56.1kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 0.150nMAssay Description:Tested in vitro for inhibition of human Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 0.190nM ΔG°: -54.9kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Displacement of [3H]CP-55940 from CB2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.200nM ΔG°: -54.8kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 0.240nMAssay Description:Displacement of [3H]CP-55940 from CB1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.25nM ΔG°: -54.3kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 0.260nM ΔG°: -54.2kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 0.270nM ΔG°: -54.1kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 0.270nM ΔG°: -54.1kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 0.290nM ΔG°: -53.9kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Tested in vitro for inhibition of rabbit Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Inhibition of EGFR T790M/del746 to 750 mutant (unknown origin) by high-throughput biochemical screeningMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3...More data for this Ligand-Target Pair
Affinity DataKi: 0.310nM ΔG°: -53.7kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 0.350nMAssay Description:Inhibition of human EGFR T790M/del (746 to 750 residues) mutant assessed as apparent inhibition constant using Fl-EEPLYWSFPAKKK-CONH2 peptide as subs...More data for this Ligand-Target Pair
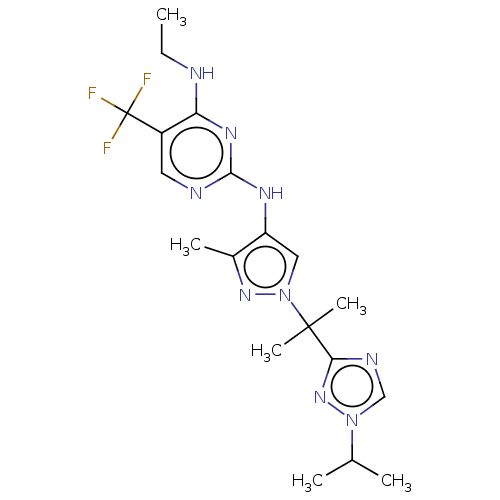
Ligand Info
Affinity DataKi: 0.380nM ΔG°: -53.2kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 0.420nM ΔG°: -53.0kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 0.460nMAssay Description:Tested in vitro for inhibition of human Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3...More data for this Ligand-Target Pair
Affinity DataKi: >0.530nMAssay Description:Inhibition of human wild type EGFR using Fl-EEPLYWSFPAKKK-CONH2 as substrate preincubated for 30 mins followed by addition of substrate measured afte...More data for this Ligand-Target Pair
Affinity DataKi: 0.540nMAssay Description:Tested in vitro for inhibition of human Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 0.580nM ΔG°: -52.2kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3...More data for this Ligand-Target Pair
Affinity DataKi: <0.600nMAssay Description:Inhibition of human EGFR T790M/L858R double mutant assessed as apparent inhibition constant using Fl-EEPLYWSFPAKKK-CONH2 as peptide substrate preincu...More data for this Ligand-Target Pair
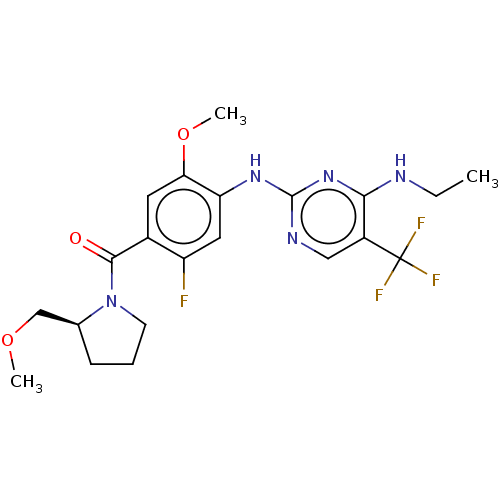
Ligand Info
Affinity DataKi: 0.600nMAssay Description:This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3...More data for this Ligand-Target Pair
Affinity DataKi: <0.600nMAssay Description:Inhibition of human EGFR T790M/L858R double mutant assessed as apparent inhibition constant using Fl-EEPLYWSFPAKKK-CONH2 as peptide substrate preincu...More data for this Ligand-Target Pair
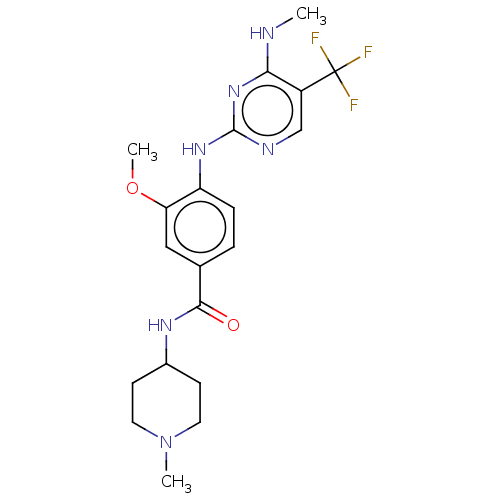
Ligand Info
Affinity DataKi: 0.600nMAssay Description:This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.600nMAssay Description:Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3...More data for this Ligand-Target Pair
Affinity DataKi: 0.650nM ΔG°: -51.9kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:Inhibition of LRRK2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3...More data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:Inhibition of LRRK2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 0.790nMAssay Description:Inhibition of human EGFR T790M/L858R double mutant assessed as apparent inhibition constant using Fl-EEPLYWSFPAKKK-CONH2 as peptide substrate preincu...More data for this Ligand-Target Pair
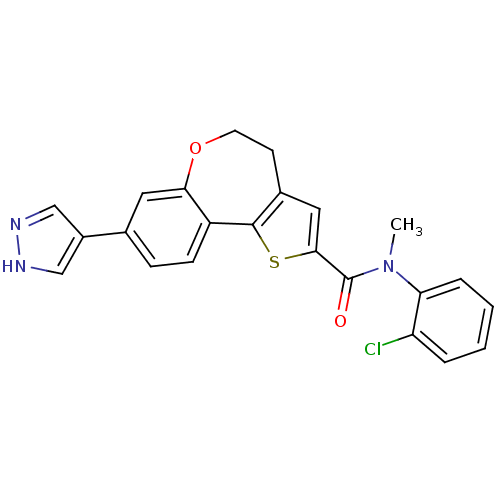
Ligand Info



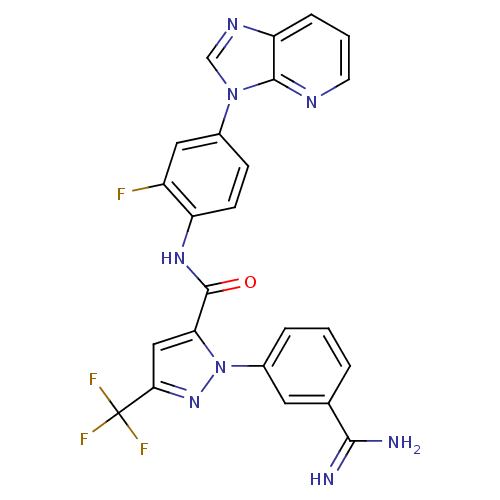


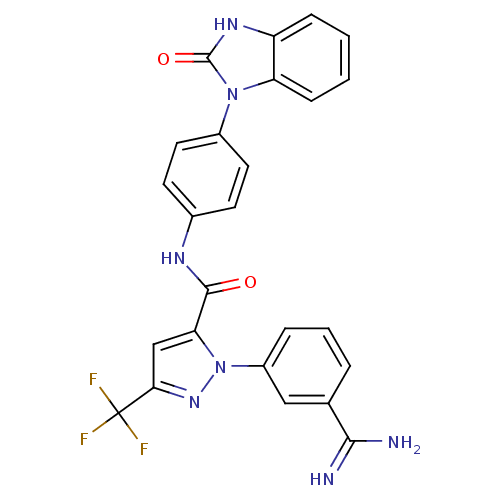

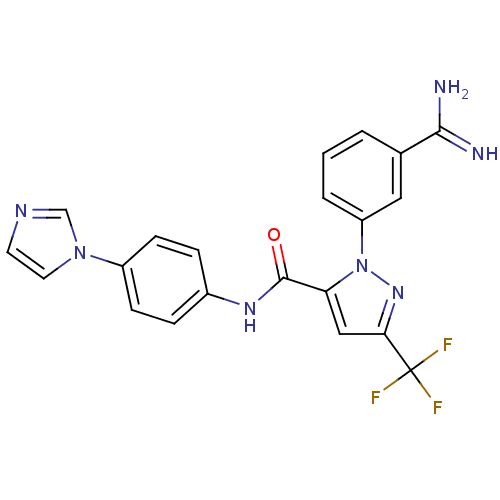


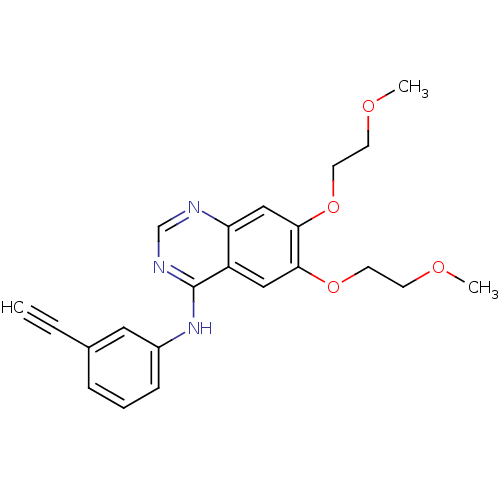
 3D Structure (crystal)
3D Structure (crystal)