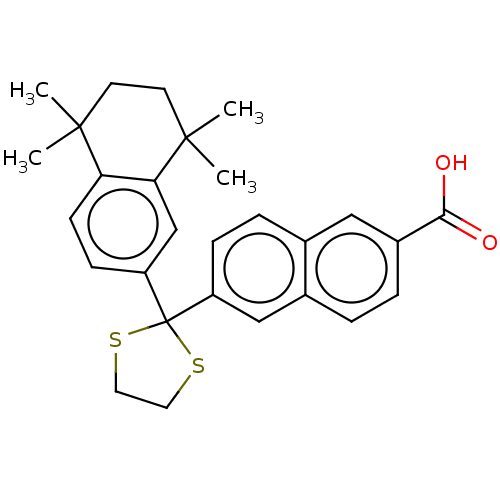
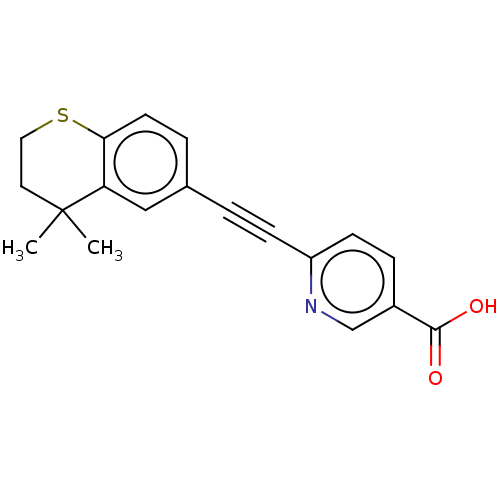
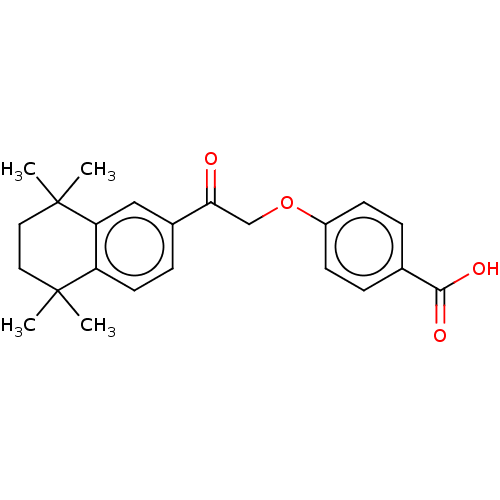
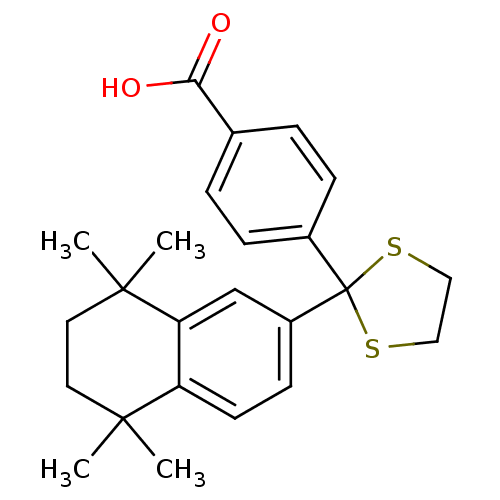
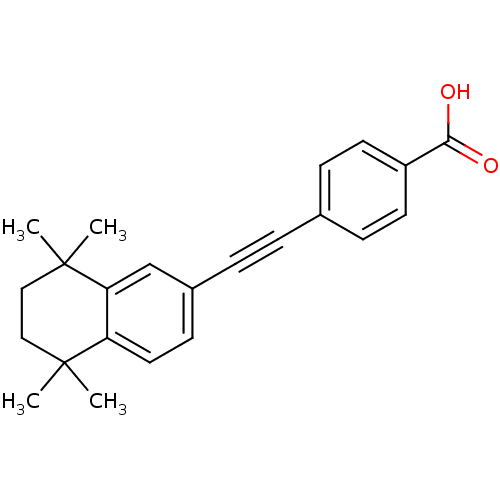
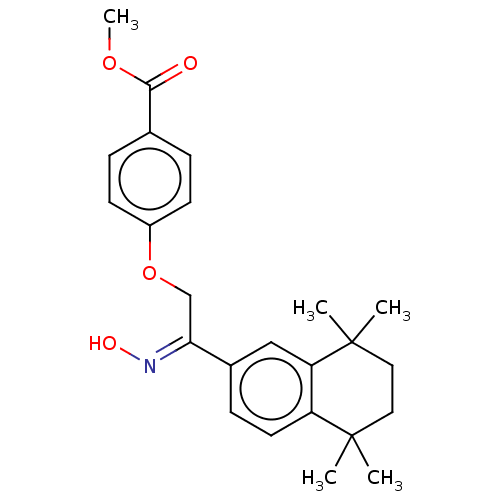
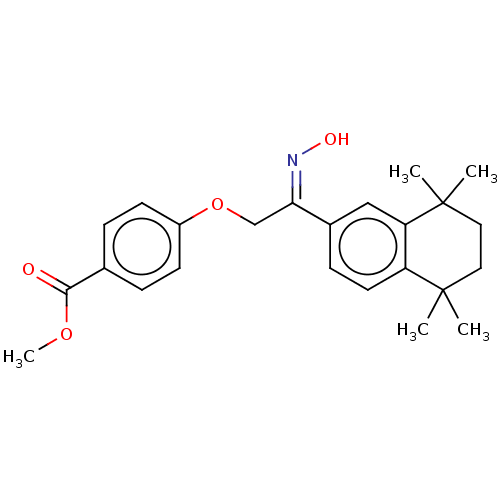
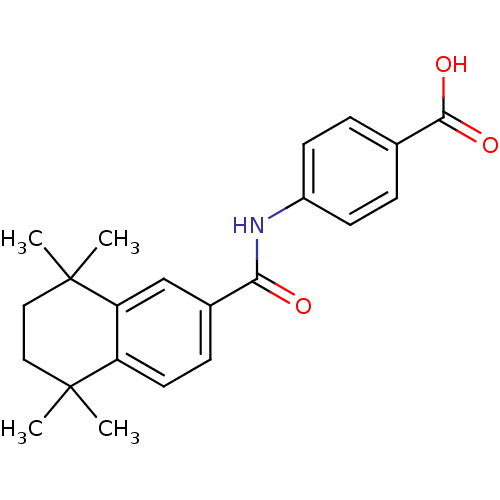
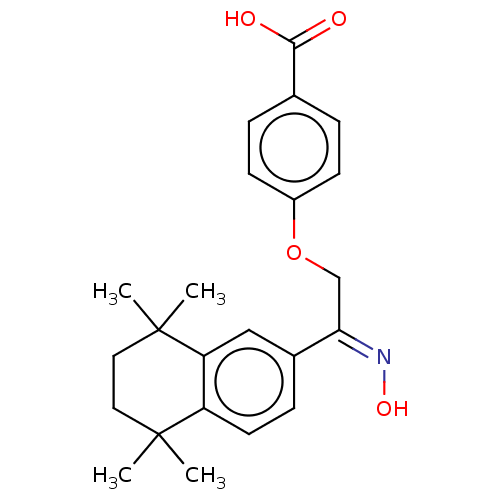
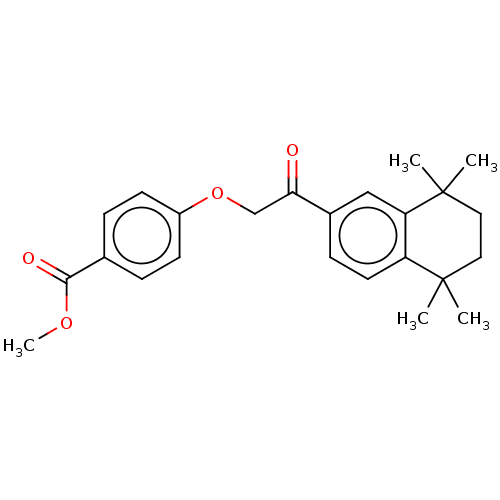
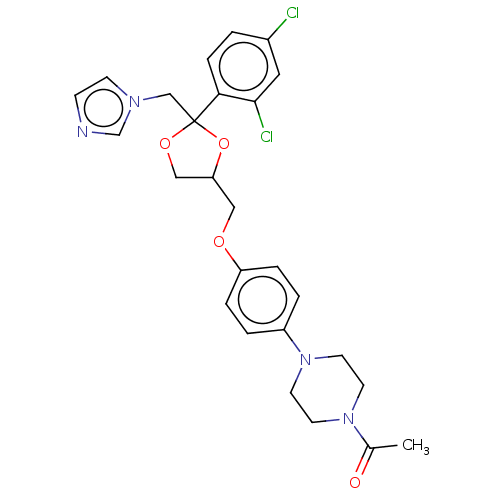
TargetCytochrome P450 26B1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 0.460nMAssay Description:Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen...More data for this Ligand-Target Pair
TargetCytochrome P450 26B1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 0.460nMAssay Description:Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit...More data for this Ligand-Target Pair
TargetCytochrome P450 26B1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 3.10nMAssay Description:Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen...More data for this Ligand-Target Pair
TargetCytochrome P450 26A1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 4.30nMAssay Description:Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen...More data for this Ligand-Target Pair
TargetCytochrome P450 26A1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 5.10nMAssay Description:Inhibition of microsomal fraction of human CYP26A1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit...More data for this Ligand-Target Pair
TargetCytochrome P450 26A1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 5.10nMAssay Description:Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen...More data for this Ligand-Target Pair
TargetCytochrome P450 26B1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 18nMAssay Description:Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit...More data for this Ligand-Target Pair
TargetCytochrome P450 26B1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 18nMAssay Description:Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen...More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: <20nMAssay Description:Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ...More data for this Ligand-Target Pair
TargetCytochrome P450 26A1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 60nMAssay Description:Inhibition of microsomal fraction of human CYP26A1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit...More data for this Ligand-Target Pair
TargetCytochrome P450 26A1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 110nMAssay Description:Inhibition of microsomal fraction of human CYP26A1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit...More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 120nMAssay Description:Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ...More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 140nMAssay Description:Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ...More data for this Ligand-Target Pair
TargetCytochrome P450 26B1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 140nMAssay Description:Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen...More data for this Ligand-Target Pair
TargetCytochrome P450 2C8(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 220nMAssay Description:Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ...More data for this Ligand-Target Pair
TargetCytochrome P450 26A1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 240nMAssay Description:Inhibition of microsomal fraction of human CYP26A1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit...More data for this Ligand-Target Pair
TargetCytochrome P450 26A1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 270nMAssay Description:Inhibition of microsomal fraction of human CYP26A1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit...More data for this Ligand-Target Pair
TargetCytochrome P450 26A1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 340nMAssay Description:Inhibition of microsomal fraction of human CYP26A1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit...More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 470nMAssay Description:Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ...More data for this Ligand-Target Pair
TargetCytochrome P450 2C8(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 480nMAssay Description:Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ...More data for this Ligand-Target Pair
TargetCytochrome P450 26B1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 520nMAssay Description:Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit...More data for this Ligand-Target Pair
TargetCytochrome P450 26A1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 660nMAssay Description:Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen...More data for this Ligand-Target Pair
TargetCytochrome P450 26B1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 680nMAssay Description:Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit...More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 680nMAssay Description:Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ...More data for this Ligand-Target Pair
TargetCytochrome P450 26B1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 930nMAssay Description:Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit...More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 1.01E+3nMAssay Description:Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ...More data for this Ligand-Target Pair
TargetCytochrome P450 26B1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 1.03E+3nMAssay Description:Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit...More data for this Ligand-Target Pair
TargetCytochrome P450 26B1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 1.03E+3nMAssay Description:Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit...More data for this Ligand-Target Pair
TargetCytochrome P450 26B1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit...More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 1.22E+3nMAssay Description:Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ...More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 1.25E+3nMAssay Description:Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ...More data for this Ligand-Target Pair
TargetCytochrome P450 26A1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of microsomal fraction of human CYP26A1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit...More data for this Ligand-Target Pair
TargetCytochrome P450 26B1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit...More data for this Ligand-Target Pair
TargetCytochrome P450 26B1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit...More data for this Ligand-Target Pair
TargetCytochrome P450 2C8(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 1.56E+3nMAssay Description:Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ...More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 1.58E+3nMAssay Description:Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ...More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 1.63E+3nMAssay Description:Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ...More data for this Ligand-Target Pair
TargetCytochrome P450 26A1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 1.63E+3nMAssay Description:Inhibition of microsomal fraction of human CYP26A1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit...More data for this Ligand-Target Pair
TargetCytochrome P450 26A1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 1.66E+3nMAssay Description:Inhibition of microsomal fraction of human CYP26A1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit...More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 1.69E+3nMAssay Description:Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ...More data for this Ligand-Target Pair
TargetCytochrome P450 26B1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit...More data for this Ligand-Target Pair
TargetCytochrome P450 2C8(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 1.76E+3nMAssay Description:Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ...More data for this Ligand-Target Pair
TargetCytochrome P450 26A1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of microsomal fraction of human CYP26A1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit...More data for this Ligand-Target Pair
TargetCytochrome P450 26A1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 1.90E+3nMAssay Description:Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen...More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 2.30E+3nMAssay Description:Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ...More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 2.57E+3nMAssay Description:Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ...More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 2.60E+3nMAssay Description:Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ...More data for this Ligand-Target Pair
TargetCytochrome P450 26A1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of microsomal fraction of human CYP26A1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit...More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 2.95E+3nMAssay Description:Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ...More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 3.15E+3nMAssay Description:Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ...More data for this Ligand-Target Pair

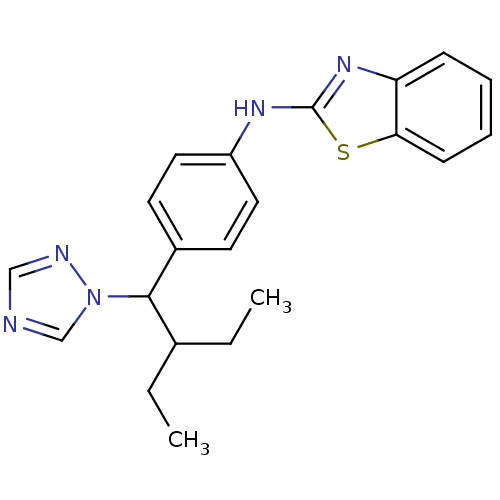
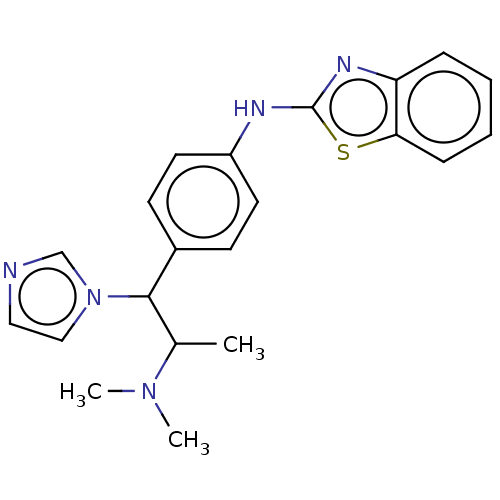

 3D Structure (crystal)
3D Structure (crystal)