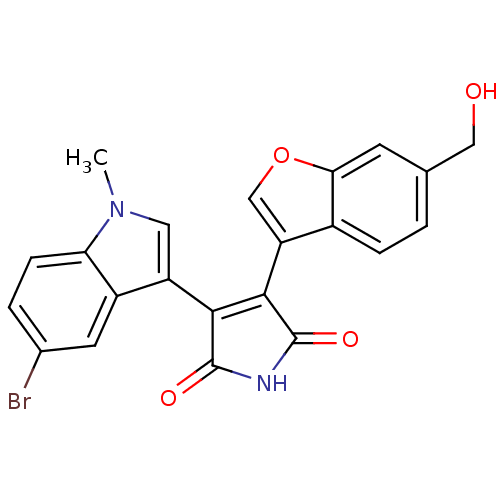
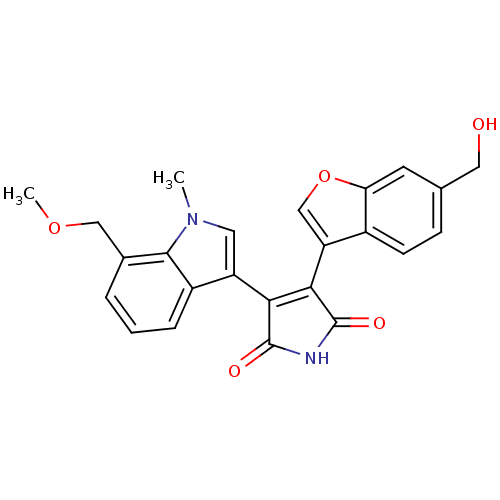
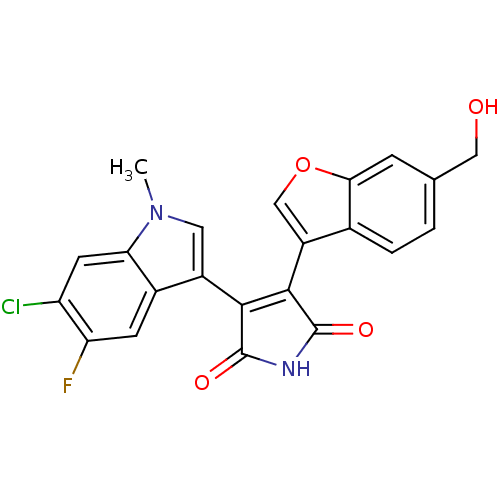
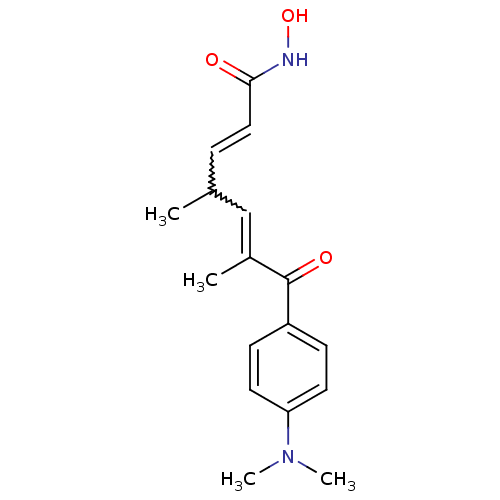
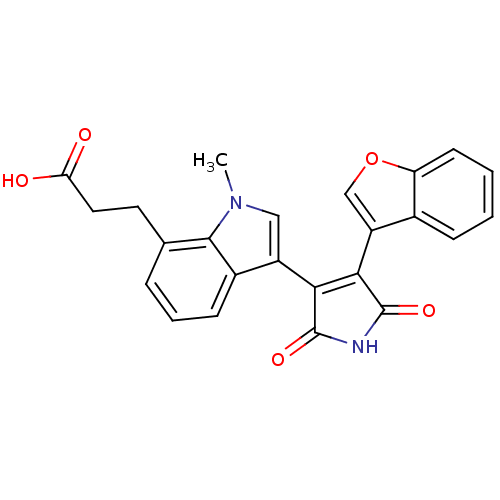
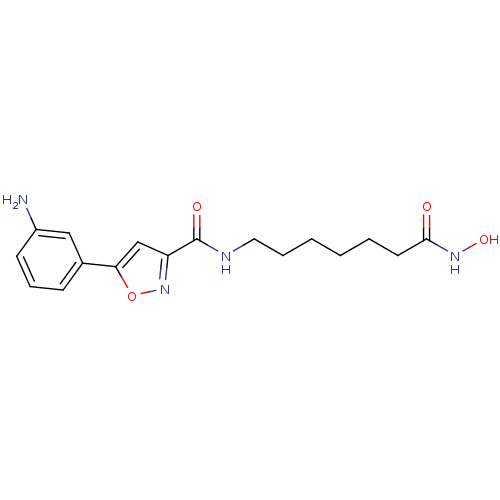
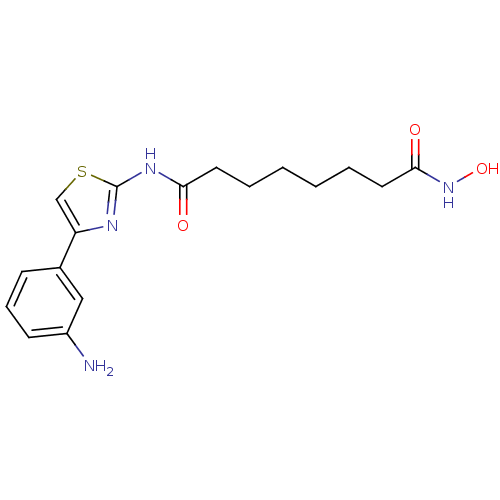
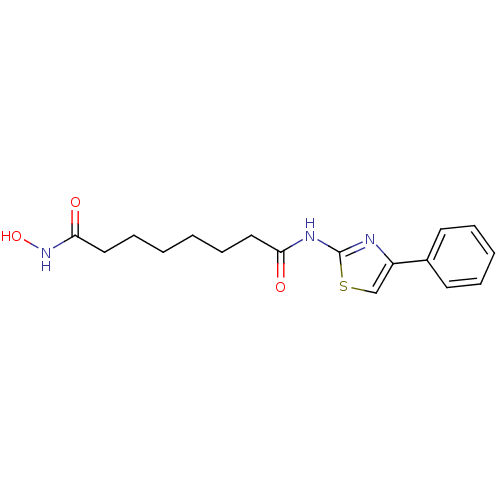
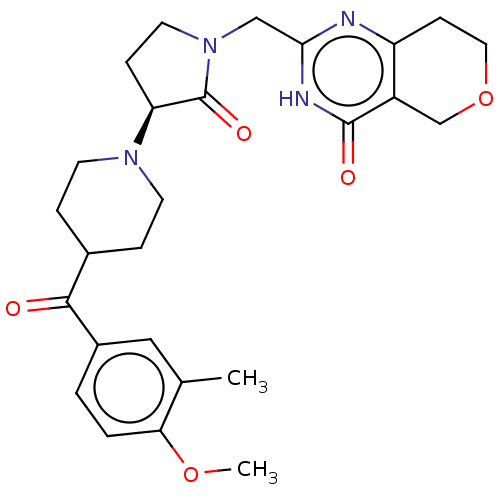
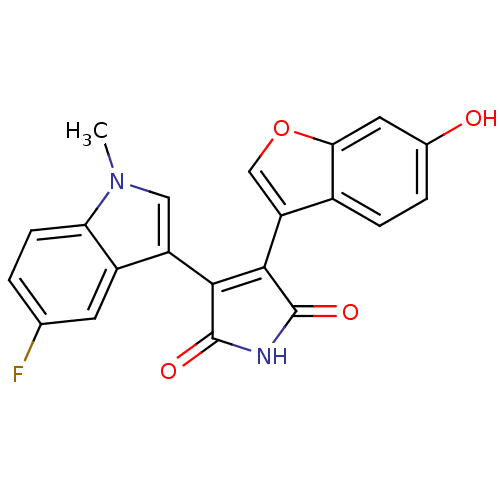
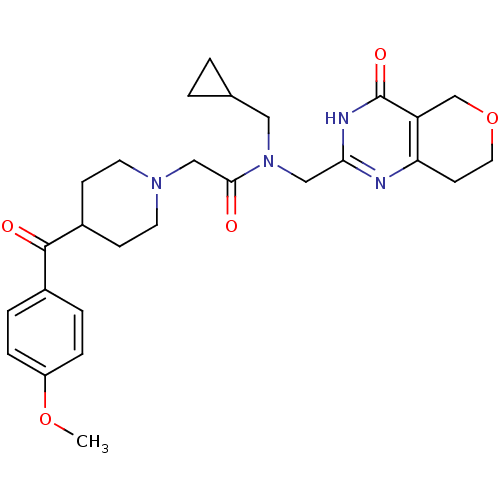
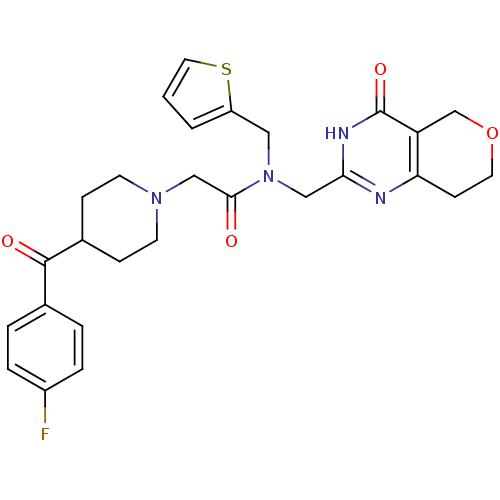
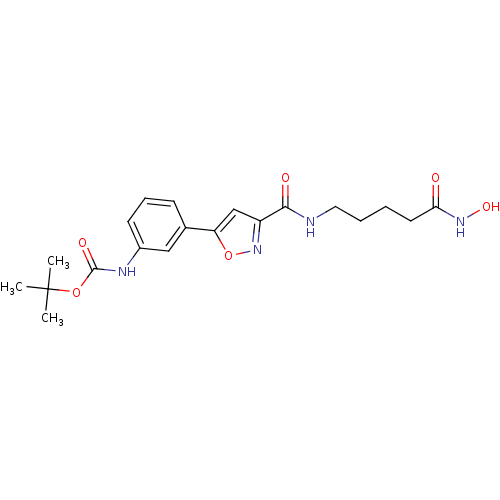
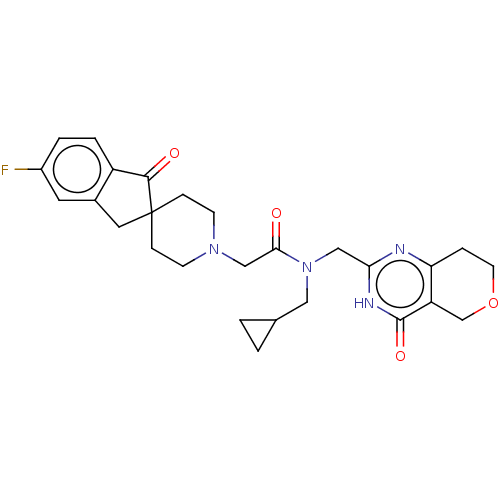
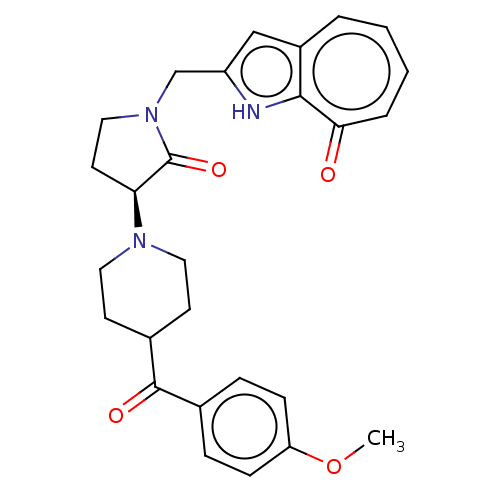
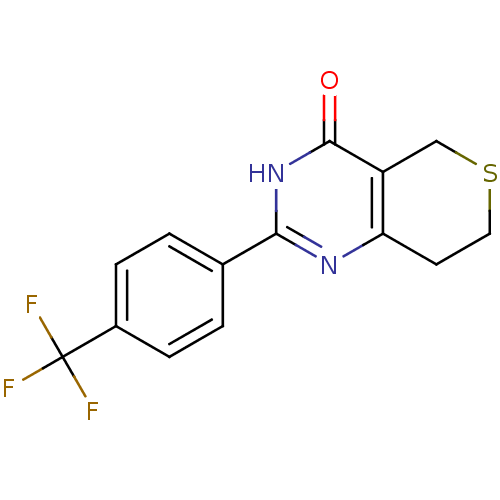
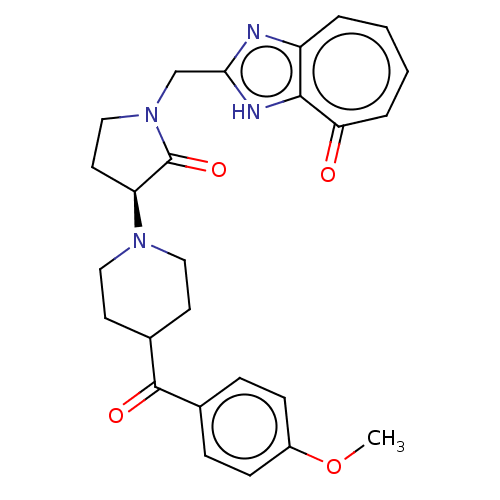
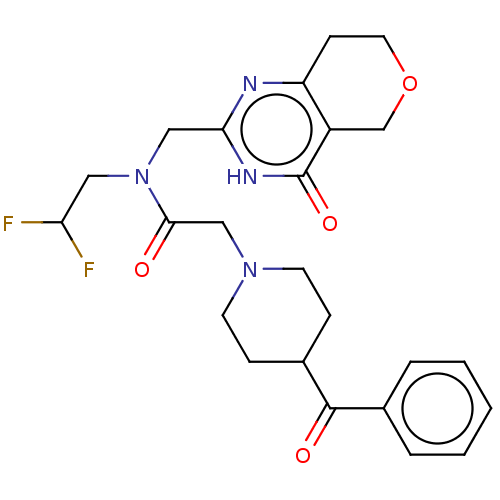
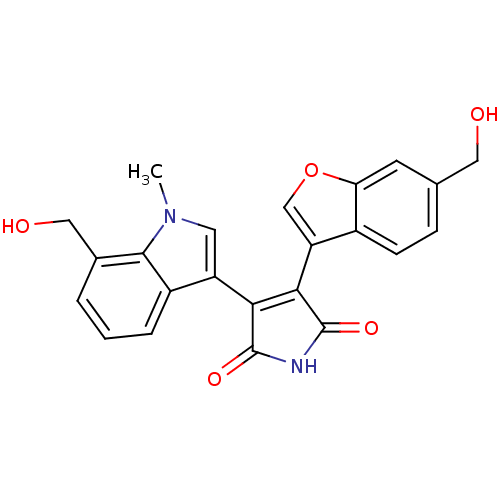
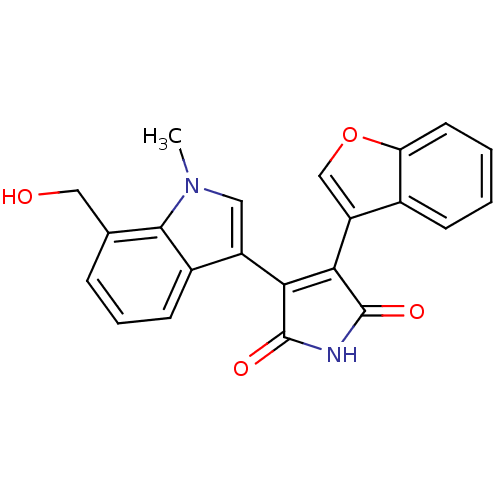
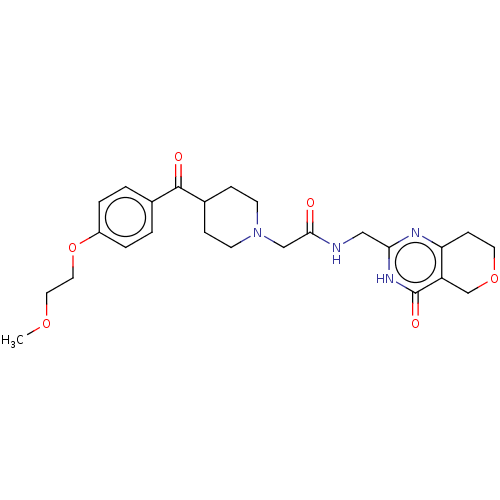
Affinity DataIC50: 0.00200nMAssay Description:Inhibition of HDAC6 (unknown origin) after 17 hrsMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 0.230nMAssay Description:Inhibition of human GSK3-beta by scintillation countingMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 0.350nMAssay Description:Inhibition of human GSK3-beta by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.420nMAssay Description:Inhibition of HDAC3 (unknown origin) after 17 hrsMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 0.510nMAssay Description:Inhibition of human GSK3-beta by scintillation countingMore data for this Ligand-Target Pair
TargetRAC-gamma serine/threonine-protein kinase(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 0.730nMAssay Description:Inhibition of human GSK3-beta by scintillation countingMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 0.950nMAssay Description:Inhibition of human GSK3-beta by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of HDAC6 (unknown origin) after 17 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMT: 2°CAssay Description:The autoparsylation activity of the TNKS 1/2 or PARP1/2 enzymes was measured by the liquid chromatography-mass spectrometry (LC/MS) detection of nico...More data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of human GSK3-beta by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 1.38nMAssay Description:Inhibition of HDAC8 (unknown origin) after 17 hrsMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Inhibition of CDK2/Cyclin AMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of HDAC3 (unknown origin) after 17 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMT: 2°CAssay Description:The autoparsylation activity of the TNKS 1/2 or PARP1/2 enzymes was measured by the liquid chromatography-mass spectrometry (LC/MS) detection of nico...More data for this Ligand-Target Pair
Affinity DataIC50: 2.54nMAssay Description:Inhibition of HDAC6 (unknown origin) after 17 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human recombinant HDAC6More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human recombinant HDAC6More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human recombinant HDAC1More data for this Ligand-Target Pair
Affinity DataIC50: 3nMT: 2°CAssay Description:The human tankyrase 1 PARP catalytic domain, TNKS1P, was cloned into a pDONR221 vector using the Invitrogen Gateway Technology. This entry clone was ...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:The human tankyrase 1 PARP catalytic domain, TNKS1P, was cloned into a pDONR221 vector using the Invitrogen Gateway Technology. This entry clone was ...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 1(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 3.30nMAssay Description:Inhibition of CDK1/Cyclin BMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 9(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 3.30nMAssay Description:Inhibition of CDK9/cyclin T1More data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 3.5nMAssay Description:Inhibition of human GSK3-beta by scintillation countingMore data for this Ligand-Target Pair
TargetProtein Wnt-3a(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3.5nMAssay Description:Inhibition of WNT3A signaling in HEK293 cells by luciferase reporter gene assay in presence of forskolinMore data for this Ligand-Target Pair
TargetProtein Wnt-3a(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3.60nMAssay Description:Inhibition of WNT3A signaling in HEK293 cells by luciferase reporter gene assay in presence of forskolinMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 5(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 3.80nMAssay Description:Inhibition of CDK5/P35More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 3.80nMAssay Description:Inhibition of CDK2/Cyclin E (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.98nMAssay Description:Inhibition of HDAC6 (unknown origin) after 17 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of HDAC1 (unknown origin) after 17 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of human recombinant HDAC1More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 5(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of CDK5/P25 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 4nMT: 2°CAssay Description:The autoparsylation activity of the TNKS 1/2 or PARP1/2 enzymes was measured by the liquid chromatography-mass spectrometry (LC/MS) detection of nico...More data for this Ligand-Target Pair
Affinity DataIC50: 4.04nMAssay Description:Inhibition of HDAC3 (unknown origin) after 17 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The human tankyrase 1 PARP catalytic domain, TNKS1P, was cloned into a pDONR221 vector using the Invitrogen Gateway Technology. This entry clone was ...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of GST-tagged TNKS2P catalytic domain autoPARsylation measuring nicotinamide concentration after 2 hrs by LC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMT: 2°CAssay Description:The human tankyrase 1 PARP catalytic domain, TNKS1P, was cloned into a pDONR221 vector using the Invitrogen Gateway Technology. This entry clone was ...More data for this Ligand-Target Pair
TargetPolyamine deacetylase HDAC10(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of HDAC10 (unknown origin) after 17 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human recombinant HDAC6More data for this Ligand-Target Pair
Affinity DataIC50: 5nMT: 2°CAssay Description:The autoparsylation activity of the TNKS 1/2 or PARP1/2 enzymes was measured by the liquid chromatography-mass spectrometry (LC/MS) detection of nico...More data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 5.10nMAssay Description:Inhibition of human GSK3-beta by scintillation countingMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 5.40nMAssay Description:Inhibition of human GSK3-beta by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 5.86nMAssay Description:Inhibition of HDAC6 (unknown origin) after 17 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:The human tankyrase 1 PARP catalytic domain, TNKS1P, was cloned into a pDONR221 vector using the Invitrogen Gateway Technology. This entry clone was ...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:The human tankyrase 1 PARP catalytic domain, TNKS1P, was cloned into a pDONR221 vector using the Invitrogen Gateway Technology. This entry clone was ...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMT: 2°CAssay Description:The human tankyrase 1 PARP catalytic domain, TNKS1P, was cloned into a pDONR221 vector using the Invitrogen Gateway Technology. This entry clone was ...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMT: 2°CAssay Description:The human tankyrase 1 PARP catalytic domain, TNKS1P, was cloned into a pDONR221 vector using the Invitrogen Gateway Technology. This entry clone was ...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMT: 2°CAssay Description:The autoparsylation activity of the TNKS 1/2 or PARP1/2 enzymes was measured by the liquid chromatography-mass spectrometry (LC/MS) detection of nico...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMT: 2°CAssay Description:The autoparsylation activity of the TNKS 1/2 or PARP1/2 enzymes was measured by the liquid chromatography-mass spectrometry (LC/MS) detection of nico...More data for this Ligand-Target Pair


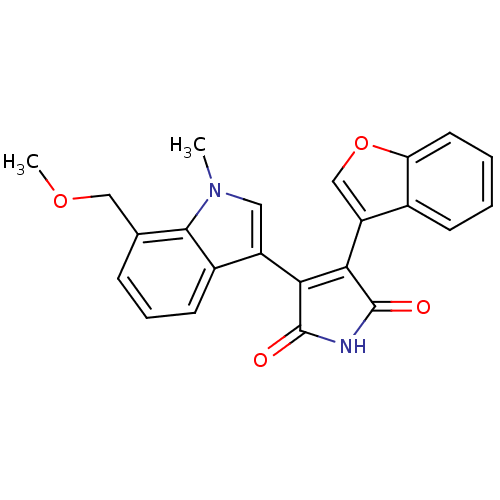
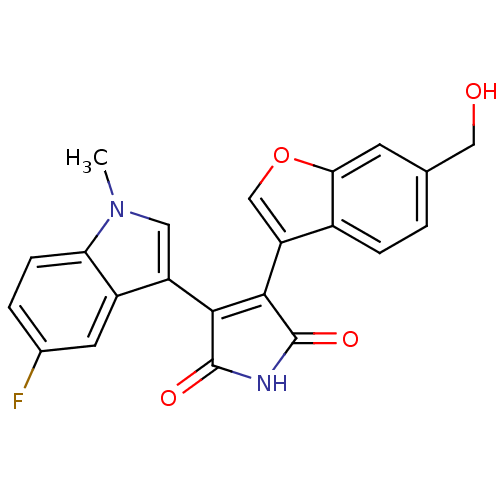
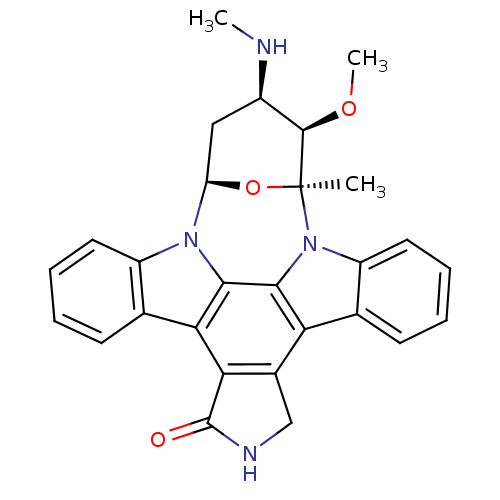
 3D Structure (crystal)
3D Structure (crystal)