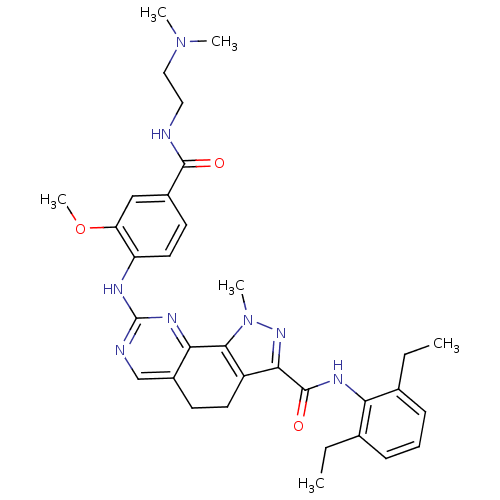
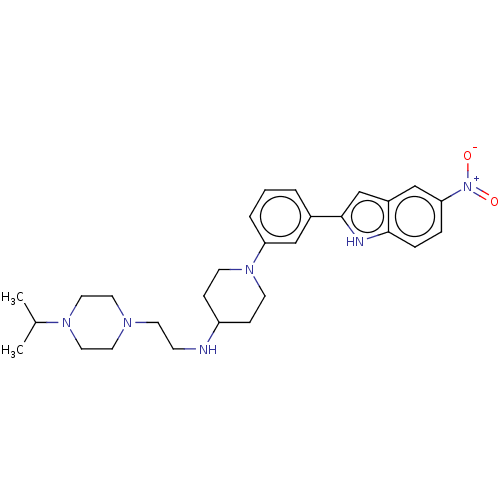
Affinity DataKi: 0.0120nMAssay Description:Inhibition of human acetylcholine esteraseMore data for this Ligand-Target Pair
Affinity DataKi: 0.0600nMAssay Description:Inhibition of human butyrylcholine esteraseMore data for this Ligand-Target Pair
Affinity DataKi: 0.230nMAssay Description:Inhibition of human acetylcholine esteraseMore data for this Ligand-Target Pair
Affinity DataKi: 0.780nMAssay Description:Inhibition of human acetylcholine esteraseMore data for this Ligand-Target Pair
Affinity DataKi: 0.820nMAssay Description:Inhibition of human butyrylcholine esteraseMore data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Inhibition of human acetylcholine esteraseMore data for this Ligand-Target Pair
Affinity DataKi: 1.70nMAssay Description:Inhibition of human butyrylcholine esteraseMore data for this Ligand-Target Pair
Affinity DataKi: 1.90nMAssay Description:Inhibition of human acetylcholine esteraseMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Inhibition of human acetylcholine esteraseMore data for this Ligand-Target Pair
Affinity DataKi: 8.30nMAssay Description:Inhibition of human butyrylcholine esteraseMore data for this Ligand-Target Pair
Affinity DataKi: 21nMAssay Description:Inhibition of human butyrylcholine esteraseMore data for this Ligand-Target Pair
Affinity DataKi: 28nMAssay Description:Inhibition of human acetylcholine esteraseMore data for this Ligand-Target Pair
Affinity DataKi: 41nMAssay Description:Inhibition of human butyrylcholine esteraseMore data for this Ligand-Target Pair
Affinity DataKi: 62nMAssay Description:Inhibition of human butyrylcholine esteraseMore data for this Ligand-Target Pair
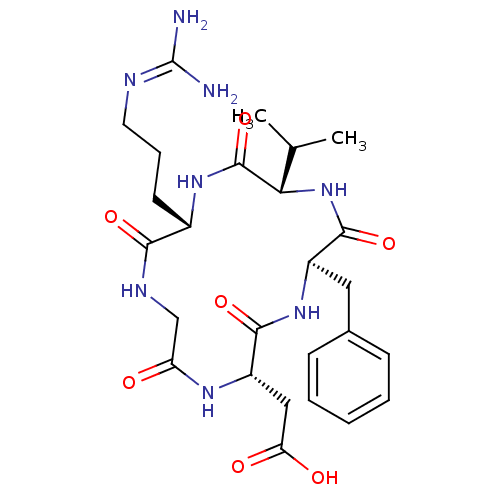
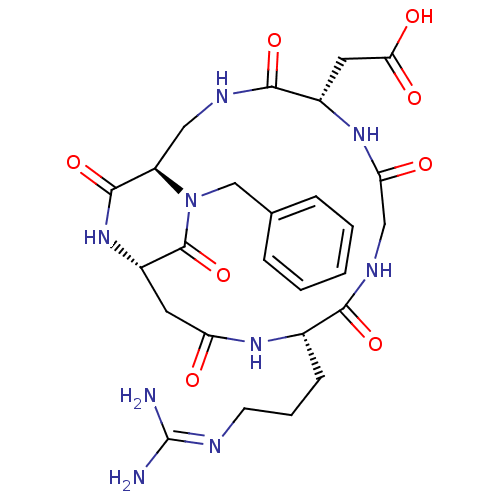
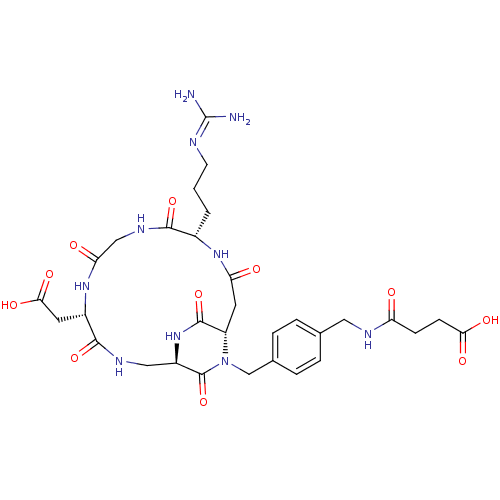
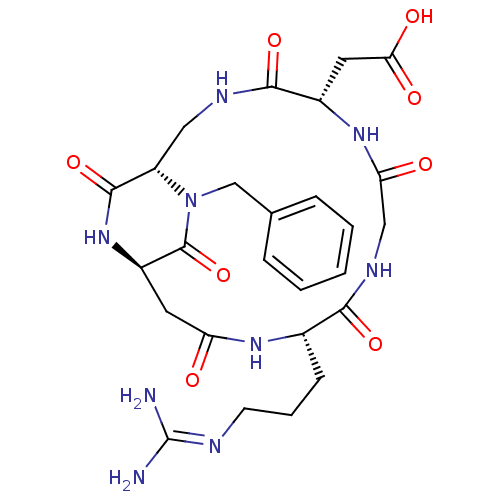
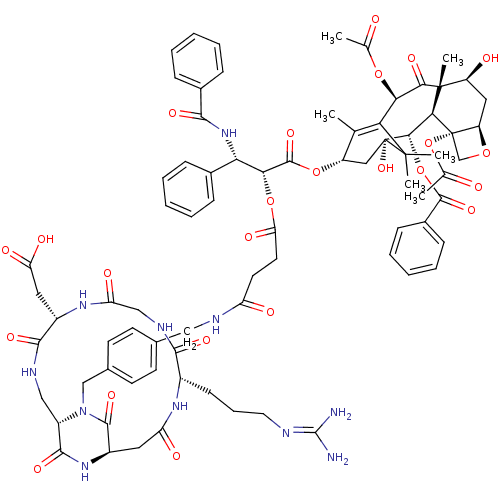
Affinity DataIC50: 0.200nMAssay Description:Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta5 receptor after 3 hrs by microplate reader analysisMore data for this Ligand-Target Pair
TargetCyclin-A2/Cyclin-dependent kinase 2(Homo sapiens (Human))
Nerviano Medical Sciences
Curated by ChEMBL
Nerviano Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of CDK2/Cyclin A assessed as [33P]-gamma-ATP incorporation into substrate by gamma countingMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysisMore data for this Ligand-Target Pair
TargetCyclin-A2/Cyclin-dependent kinase 2(Homo sapiens (Human))
Nerviano Medical Sciences
Curated by ChEMBL
Nerviano Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of CDK2/Cyclin A assessed as [33P]-gamma-ATP incorporation into substrate by gamma countingMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 3.90nMAssay Description:Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 4.10nMAssay Description:Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description:Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 5.20nMAssay Description:Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 7.5nMAssay Description:Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta5 receptor after 3 hrs by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 7.60nMAssay Description:Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 8.5nMAssay Description:Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysisMore data for this Ligand-Target Pair
TargetTransitional endoplasmic reticulum ATPase(Homo sapiens (Human))
University of Pittsburgh
US Patent
University of Pittsburgh
US Patent
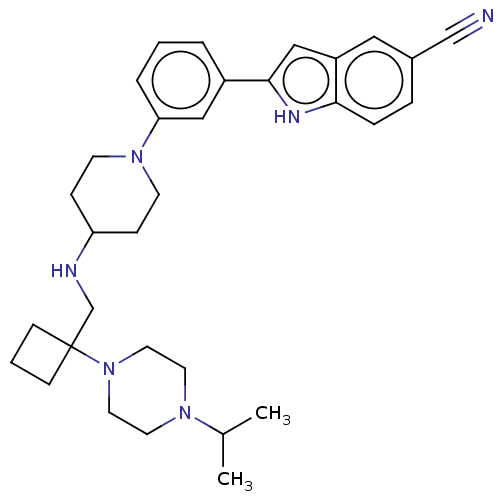
Affinity DataIC50: 15nMAssay Description:To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass...More data for this Ligand-Target Pair
TargetTransitional endoplasmic reticulum ATPase(Homo sapiens (Human))
University of Pittsburgh
US Patent
University of Pittsburgh
US Patent
Affinity DataIC50: 15nMAssay Description:To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase PLK1(Homo sapiens (Human))
Nerviano Medical Sciences
Curated by ChEMBL
Nerviano Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Inhibition of PLK1 assessed as [33P]-gamma-ATP incorporation into substrate by gamma countingMore data for this Ligand-Target Pair
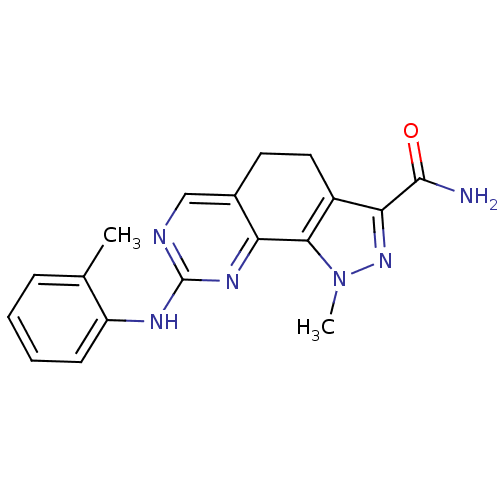
TargetDual specificity protein kinase TTK(Homo sapiens (Human))
Nerviano Medical Sciences
Curated by ChEMBL
Nerviano Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 19nMAssay Description:Inhibition of MPS1 assessed as [33P]-gamma-ATP incorporation into substrate P38-betatide by gamma countingMore data for this Ligand-Target Pair
TargetCyclin-A2/Cyclin-dependent kinase 2(Homo sapiens (Human))
Nerviano Medical Sciences
Curated by ChEMBL
Nerviano Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 19nMAssay Description:Inhibition of CDK2/Cyclin A assessed as [33P]-gamma-ATP incorporation into substrate by gamma countingMore data for this Ligand-Target Pair
TargetTransitional endoplasmic reticulum ATPase(Homo sapiens (Human))
University of Pittsburgh
US Patent
University of Pittsburgh
US Patent
Affinity DataIC50: 20nMAssay Description:Inhibition of recombinant full length human p97 (1 to 806 residues) expressed in Escherichia coli Rosetta 2 (DE3) using 100 uM ATP as substrate after...More data for this Ligand-Target Pair
TargetDual specificity protein kinase TTK(Homo sapiens (Human))
Nerviano Medical Sciences
Curated by ChEMBL
Nerviano Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 21nMAssay Description:Inhibition of MPS1 assessed as [33P]-gamma-ATP incorporation into substrate P38-betatide by gamma countingMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta5 receptor after 3 hrs by microplate reader analysisMore data for this Ligand-Target Pair
TargetTransitional endoplasmic reticulum ATPase(Homo sapiens (Human))
University of Pittsburgh
US Patent
University of Pittsburgh
US Patent
Affinity DataIC50: 25nMAssay Description:To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass...More data for this Ligand-Target Pair
TargetTransitional endoplasmic reticulum ATPase(Homo sapiens (Human))
University of Pittsburgh
US Patent
University of Pittsburgh
US Patent
Affinity DataIC50: 26nMAssay Description:To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass...More data for this Ligand-Target Pair
TargetTransitional endoplasmic reticulum ATPase(Homo sapiens (Human))
University of Pittsburgh
US Patent
University of Pittsburgh
US Patent
Affinity DataIC50: 26nMAssay Description:To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass...More data for this Ligand-Target Pair
Affinity DataIC50: 26.4nMAssay Description:Competitive inhibition of biotinylated vitronectin to integrin alphaVbeta3 receptor after 3 hrs by microplate reader analysisMore data for this Ligand-Target Pair
TargetTransitional endoplasmic reticulum ATPase(Homo sapiens (Human))
University of Pittsburgh
US Patent
University of Pittsburgh
US Patent
Affinity DataIC50: 27nMAssay Description:To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass...More data for this Ligand-Target Pair
TargetDual specificity protein kinase TTK(Homo sapiens (Human))
Nerviano Medical Sciences
Curated by ChEMBL
Nerviano Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 33nMAssay Description:Inhibition of MPS1 assessed as [33P]-gamma-ATP incorporation into substrate P38-betatide by gamma countingMore data for this Ligand-Target Pair
TargetTransitional endoplasmic reticulum ATPase(Homo sapiens (Human))
University of Pittsburgh
US Patent
University of Pittsburgh
US Patent
Affinity DataIC50: 36nMAssay Description:To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass...More data for this Ligand-Target Pair
TargetDual specificity protein kinase TTK(Homo sapiens (Human))
Nerviano Medical Sciences
Curated by ChEMBL
Nerviano Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 36nMAssay Description:Inhibition of MPS1 assessed as [33P]-gamma-ATP incorporation into substrate P38-betatide by gamma countingMore data for this Ligand-Target Pair
TargetTransitional endoplasmic reticulum ATPase(Homo sapiens (Human))
University of Pittsburgh
US Patent
University of Pittsburgh
US Patent
Affinity DataIC50: 41nMAssay Description:To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass...More data for this Ligand-Target Pair


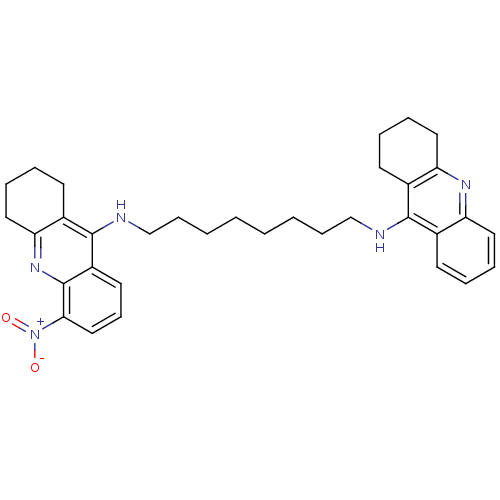
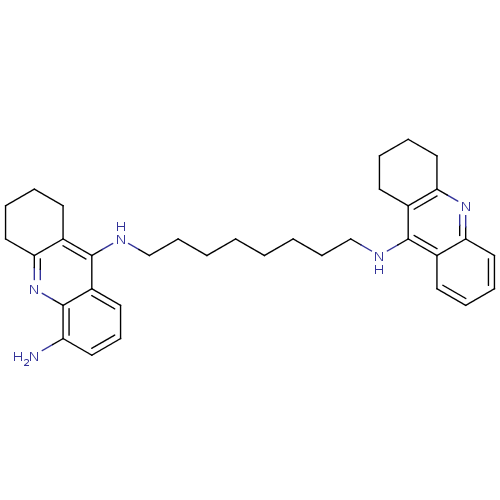
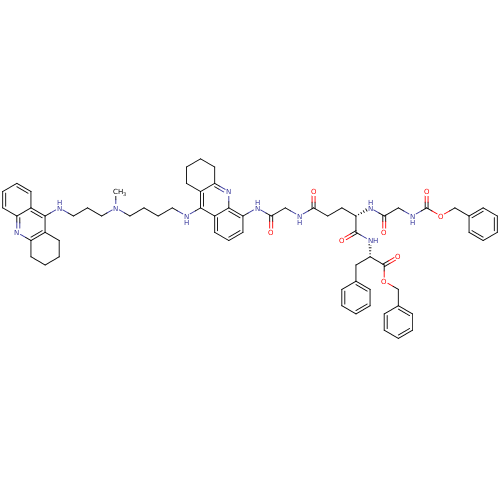
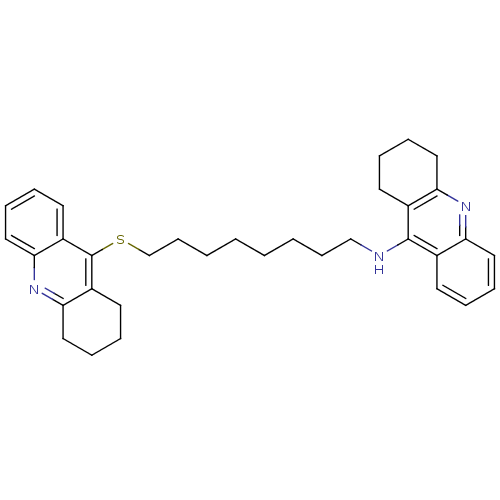


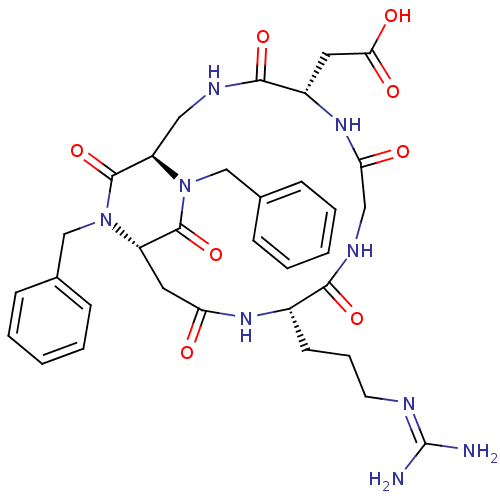

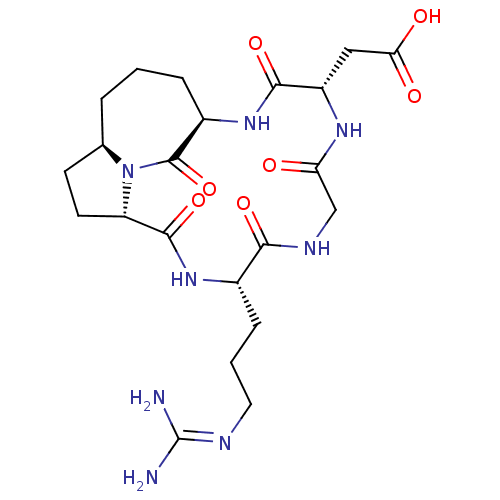
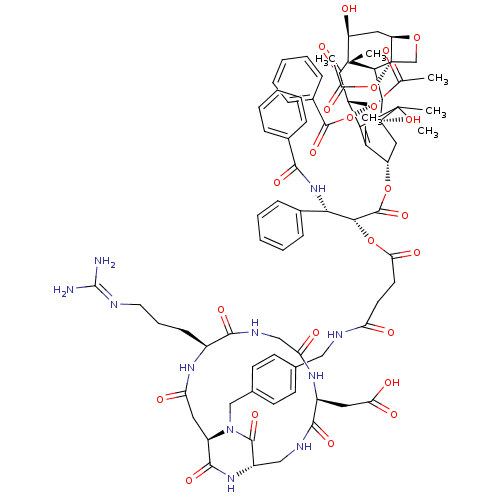
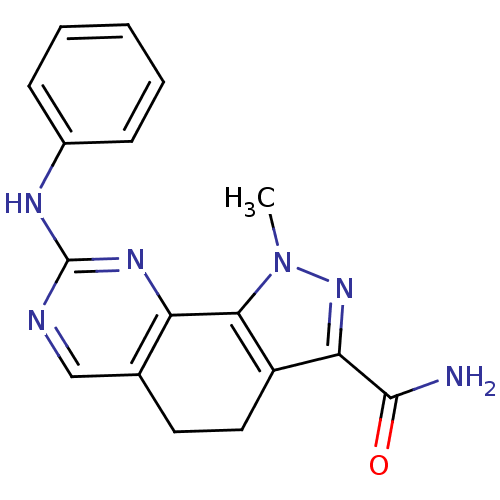
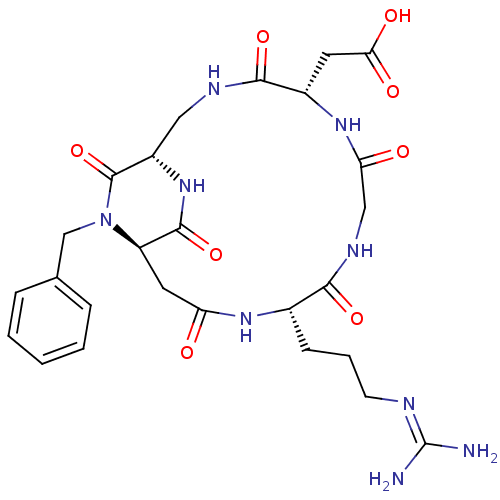



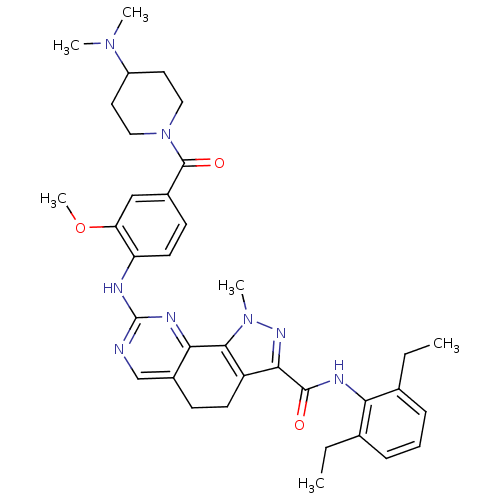
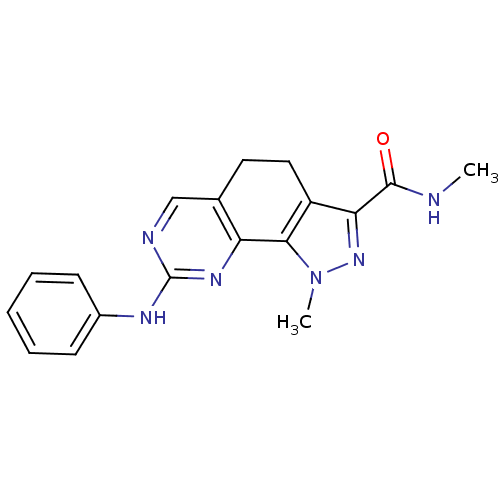
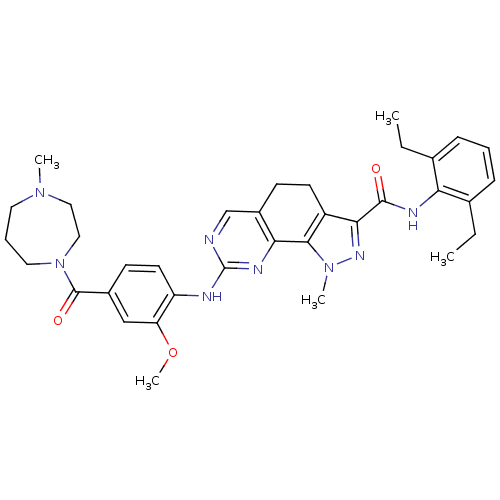

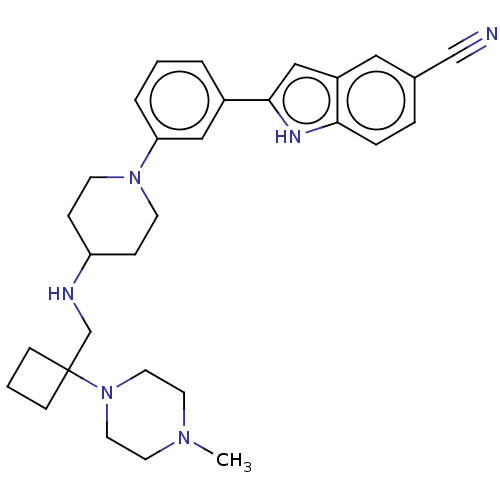

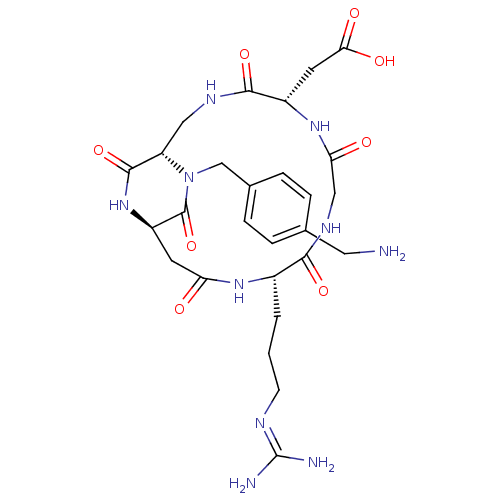

 3D Structure (crystal)
3D Structure (crystal)