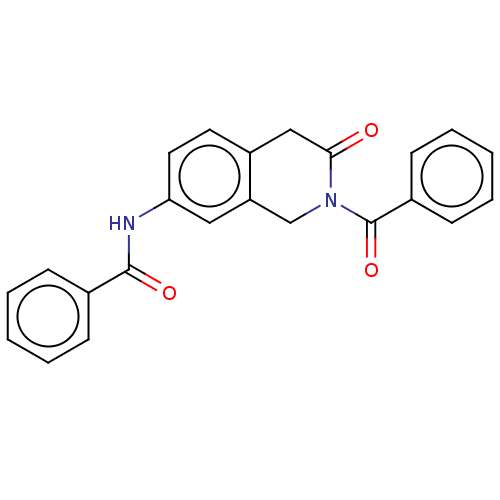
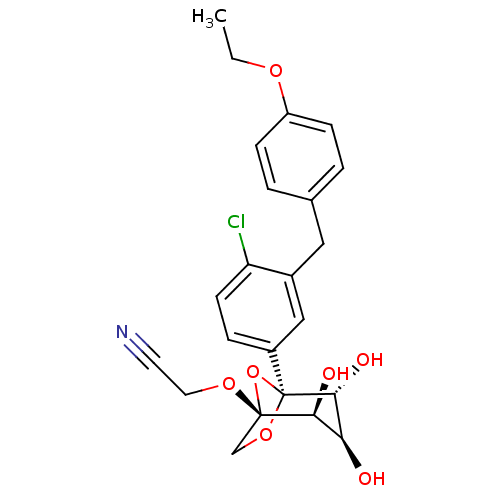
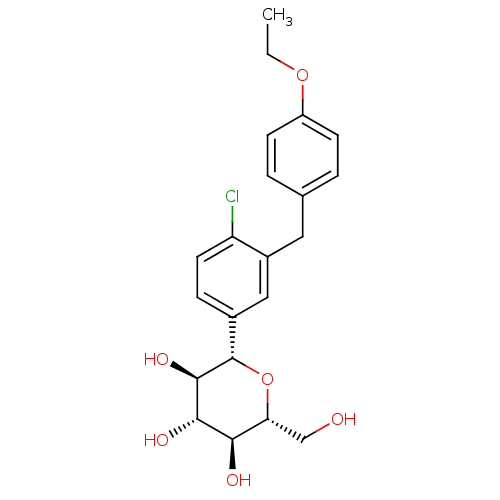
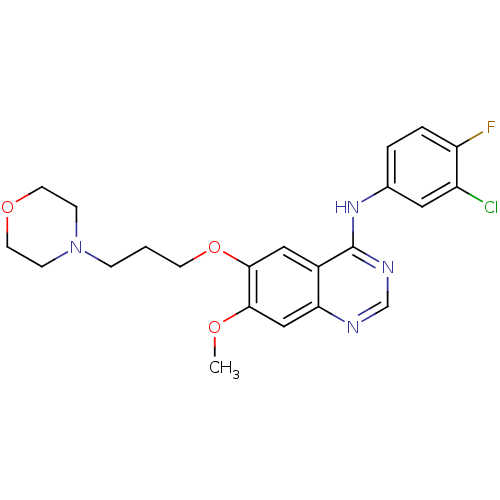
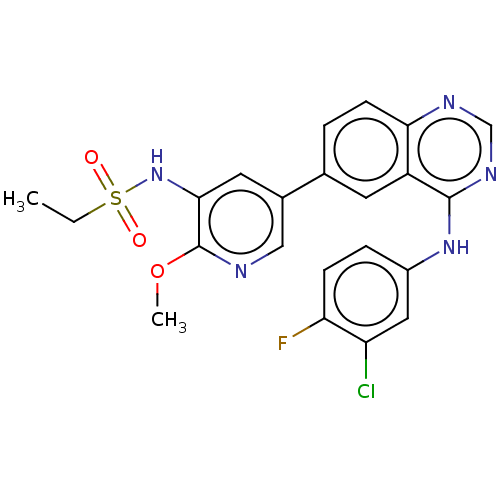
TargetUrease subunit beta(Helicobacter pylori (strain ATCC 700392 / 26695) (...)
Jishou University
Curated by ChEMBL
Jishou University
Curated by ChEMBL
Affinity DataKi: 14nMAssay Description:Mixed-type competitive inhibition of Helicobacter pylori ATCC 43504 urease using urea as substrate by Lineweaver-burk plot analysisMore data for this Ligand-Target Pair
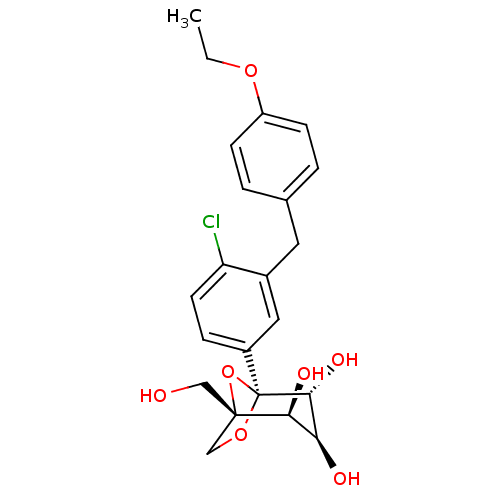
TargetUrease subunit beta(Helicobacter pylori (strain ATCC 700392 / 26695) (...)
Jishou University
Curated by ChEMBL
Jishou University
Curated by ChEMBL
Affinity DataKi: 45nMAssay Description:Mixed-type competitive inhibition of Helicobacter pylori ATCC 43504 urease using urea as substrate by Lineweaver-burk plot analysisMore data for this Ligand-Target Pair
TargetUrease subunit beta(Helicobacter pylori (strain ATCC 700392 / 26695) (...)
Jishou University
Curated by ChEMBL
Jishou University
Curated by ChEMBL
Affinity DataKi: 372nMAssay Description:Inhibition of Helicobacter pylori urease using urea as substrate assessed as inhibition constant for enzyme-urea-inhibitor complex by non-linear fitt...More data for this Ligand-Target Pair
TargetUrease subunit beta(Helicobacter pylori (strain ATCC 700392 / 26695) (...)
Jishou University
Curated by ChEMBL
Jishou University
Curated by ChEMBL
Affinity DataKi: 404nMAssay Description:Inhibition of Helicobacter pylori urease using urea as substrate assessed as inhibition constant for enzyme-inhibitor complex by non-linear fitting a...More data for this Ligand-Target Pair
TargetUrease subunit beta(Helicobacter pylori (strain ATCC 700392 / 26695) (...)
Jishou University
Curated by ChEMBL
Jishou University
Curated by ChEMBL
Affinity DataKi: 1.20E+3nMAssay Description:Inhibition of Helicobacter pylori urease using urea as substrate assessed as inhibition constant for enzyme-urea-inhibitor complex by non-linear fitt...More data for this Ligand-Target Pair
TargetUrease subunit beta(Helicobacter pylori (strain ATCC 700392 / 26695) (...)
Jishou University
Curated by ChEMBL
Jishou University
Curated by ChEMBL
Affinity DataKi: 4.74E+3nMAssay Description:Inhibition of Helicobacter pylori urease using urea as substrate assessed as inhibition constant for enzyme-inhibitor complex by non-linear fitting a...More data for this Ligand-Target Pair
TargetUrease subunit beta(Helicobacter pylori (strain ATCC 700392 / 26695) (...)
Jishou University
Curated by ChEMBL
Jishou University
Curated by ChEMBL
Affinity DataKi: 6.47E+6nMAssay Description:Substrate inhibition of Helicobacter pylori urease in presence of >4 mM urea by indophenol methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0110nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0130nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0140nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0140nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0140nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0170nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0190nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0210nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0220nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0260nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0260nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0280nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0290nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
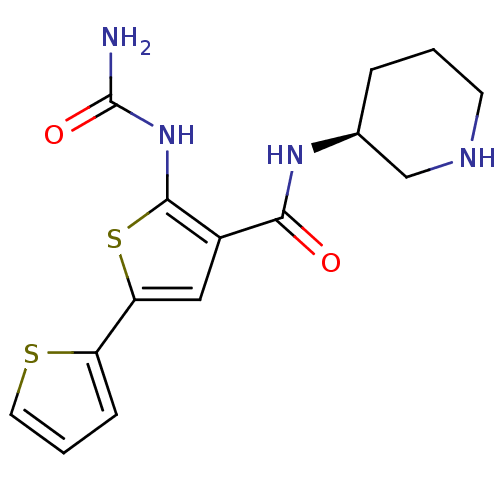
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
Astrazeneca R&D Boston
Curated by ChEMBL
Astrazeneca R&D Boston
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Inhibition of His6-CHK1 expressed in baculovirus infected Sf9 cellsMore data for this Ligand-Target Pair
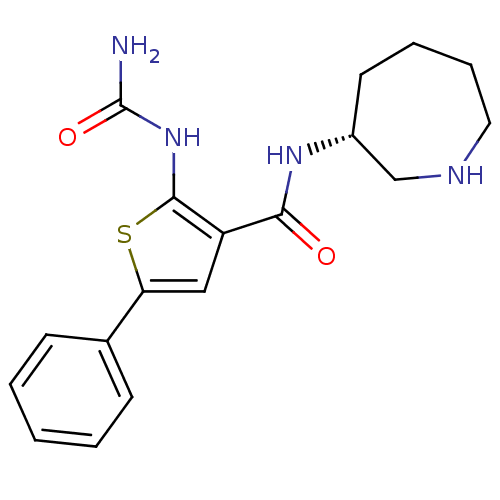
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
Astrazeneca R&D Boston
Curated by ChEMBL
Astrazeneca R&D Boston
Curated by ChEMBL
Affinity DataIC50: 0.510nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
TargetSodium/glucose cotransporter 2(Homo sapiens (Human))
Haisco Pharmaceuticals Group
Curated by ChEMBL
Haisco Pharmaceuticals Group
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of human SGLT2 expressed in CHO cells assessed as decrease in uptake of [14C]AMG after 120 mins by TopCount methodMore data for this Ligand-Target Pair
TargetSodium/glucose cotransporter 2(Homo sapiens (Human))
Haisco Pharmaceuticals Group
Curated by ChEMBL
Haisco Pharmaceuticals Group
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibition of human SGLT2 expressed in CHO cells assessed as decrease in uptake of [14C]AMG after 120 mins by TopCount methodMore data for this Ligand-Target Pair
TargetSodium/glucose cotransporter 2(Homo sapiens (Human))
Haisco Pharmaceuticals Group
Curated by ChEMBL
Haisco Pharmaceuticals Group
Curated by ChEMBL
Affinity DataIC50: 1.30nMAssay Description:Inhibition of human SGLT2 expressed in CHO cells assessed as decrease in uptake of [14C]AMG after 120 mins by TopCount methodMore data for this Ligand-Target Pair
TargetSodium/glucose cotransporter 2(Homo sapiens (Human))
Haisco Pharmaceuticals Group
Curated by ChEMBL
Haisco Pharmaceuticals Group
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of human SGLT2More data for this Ligand-Target Pair
TargetSodium/glucose cotransporter 2(Homo sapiens (Human))
Haisco Pharmaceuticals Group
Curated by ChEMBL
Haisco Pharmaceuticals Group
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of human SGLT2 expressed in CHO cells assessed as decrease in uptake of [14C]AMG after 120 mins by TopCount methodMore data for this Ligand-Target Pair
TargetSodium/glucose cotransporter 2(Homo sapiens (Human))
Haisco Pharmaceuticals Group
Curated by ChEMBL
Haisco Pharmaceuticals Group
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of human SGLT2 expressed in CHO cells assessed as decrease in uptake of [14C]AMG after 120 mins by TopCount methodMore data for this Ligand-Target Pair
TargetCaspase-3(Homo sapiens (Human))
The Fifth Affiliated Hospital Of Guangzhou Medical University
Curated by ChEMBL
The Fifth Affiliated Hospital Of Guangzhou Medical University
Curated by ChEMBL
Affinity DataIC50: 1.70nMAssay Description:Inhibition of His-tagged human recombinant caspase 3 expressed in Escherichia coli using acetyl-Asp-Glu-Val-Asp-7- amido-4-methylcoumarin (Ac-DEVD-AM...More data for this Ligand-Target Pair
TargetSodium/glucose cotransporter 2(Homo sapiens (Human))
Haisco Pharmaceuticals Group
Curated by ChEMBL
Haisco Pharmaceuticals Group
Curated by ChEMBL
Affinity DataIC50: 2.20nMAssay Description:Inhibition of human SGLT2 expressed in CHO cells assessed as decrease in uptake of [14C]AMG after 120 mins by TopCount methodMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Shenyang Pharmaceutical University
Curated by ChEMBL
Shenyang Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 2.30nMAssay Description:Inhibition of EGFR (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by substrate addition and measured after...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Shenyang Pharmaceutical University
Curated by ChEMBL
Shenyang Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 2.40nMAssay Description:Inhibition of EGFR (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by substrate addition and measured after...More data for this Ligand-Target Pair
TargetSodium/glucose cotransporter 2(Homo sapiens (Human))
Haisco Pharmaceuticals Group
Curated by ChEMBL
Haisco Pharmaceuticals Group
Curated by ChEMBL
Affinity DataIC50: 2.40nMAssay Description:Inhibition of human SGLT2 expressed in CHO cells assessed as decrease in uptake of [14C]AMG after 120 mins by TopCount methodMore data for this Ligand-Target Pair
TargetSodium/glucose cotransporter 2(Homo sapiens (Human))
Haisco Pharmaceuticals Group
Curated by ChEMBL
Haisco Pharmaceuticals Group
Curated by ChEMBL
Affinity DataIC50: 2.5nMAssay Description:Inhibition of human SGLT2 expressed in CHO cells assessed as decrease in uptake of [14C]AMG after 120 mins by TopCount methodMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
Astrazeneca R&D Boston
Curated by ChEMBL
Astrazeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
Astrazeneca R&D Boston
Curated by ChEMBL
Astrazeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
Astrazeneca R&D Boston
Curated by ChEMBL
Astrazeneca R&D Boston
Curated by ChEMBL
TargetSodium/glucose cotransporter 2(Homo sapiens (Human))
Haisco Pharmaceuticals Group
Curated by ChEMBL
Haisco Pharmaceuticals Group
Curated by ChEMBL
Affinity DataIC50: 3.40nMAssay Description:Inhibition of human SGLT2 expressed in CHO cells assessed as decrease in uptake of [14C]AMG after 120 mins by TopCount methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMAssay Description:Inhibition of human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
Astrazeneca R&D Boston
Curated by ChEMBL
Astrazeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
Astrazeneca R&D Boston
Curated by ChEMBL
Astrazeneca R&D Boston
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of His6-CHK1 expressed in baculovirus infected Sf9 cellsMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
Astrazeneca R&D Boston
Curated by ChEMBL
Astrazeneca R&D Boston
Curated by ChEMBL
TargetSodium/glucose cotransporter 2(Homo sapiens (Human))
Haisco Pharmaceuticals Group
Curated by ChEMBL
Haisco Pharmaceuticals Group
Curated by ChEMBL
Affinity DataIC50: 4.40nMAssay Description:Inhibition of human SGLT2 expressed in CHO cells assessed as decrease in uptake of [14C]AMG after 120 mins by TopCount methodMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
Astrazeneca R&D Boston
Curated by ChEMBL
Astrazeneca R&D Boston
Curated by ChEMBL
TargetSodium/glucose cotransporter 2(Homo sapiens (Human))
Haisco Pharmaceuticals Group
Curated by ChEMBL
Haisco Pharmaceuticals Group
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of human SGLT2 expressed in CHO cells assessed as decrease in uptake of [14C]AMG after 120 mins by TopCount methodMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
Astrazeneca R&D Boston
Curated by ChEMBL
Astrazeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
Astrazeneca R&D Boston
Curated by ChEMBL
Astrazeneca R&D Boston
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of CHK1 (unknown origin)More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
Astrazeneca R&D Boston
Curated by ChEMBL
Astrazeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
Astrazeneca R&D Boston
Curated by ChEMBL
Astrazeneca R&D Boston
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of human recombinant CHK1 expressed in insect cells using biotinylaminohexanoyl-KKVSRSGLYRSPMPENLNRPR as substrate after 2 hrs by scintill...More data for this Ligand-Target Pair



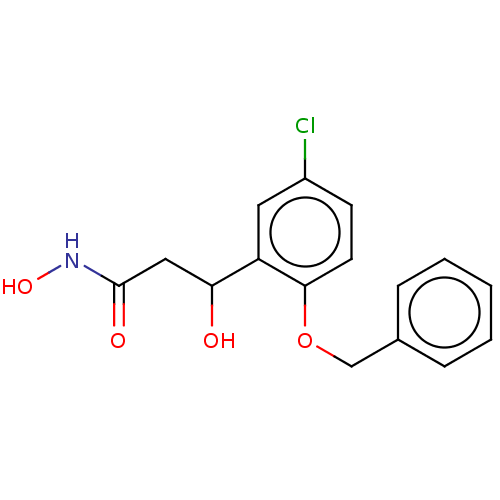
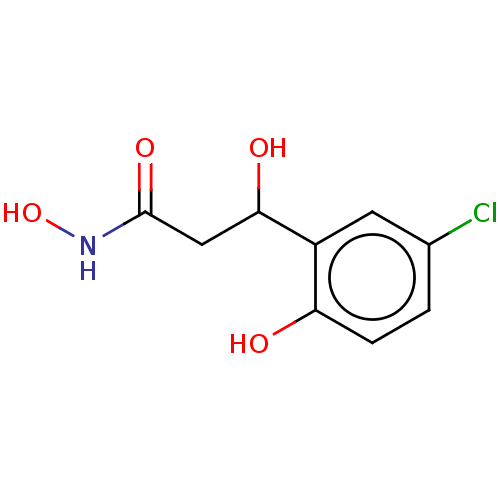

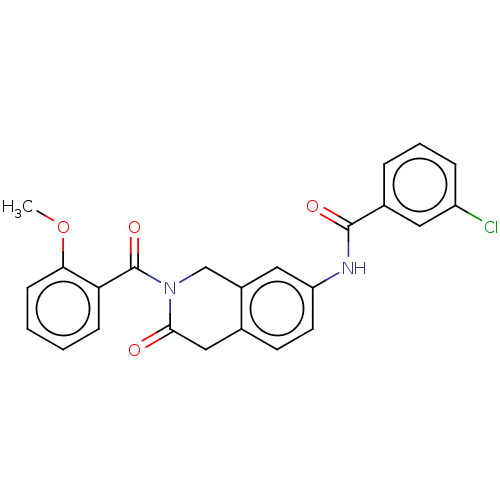


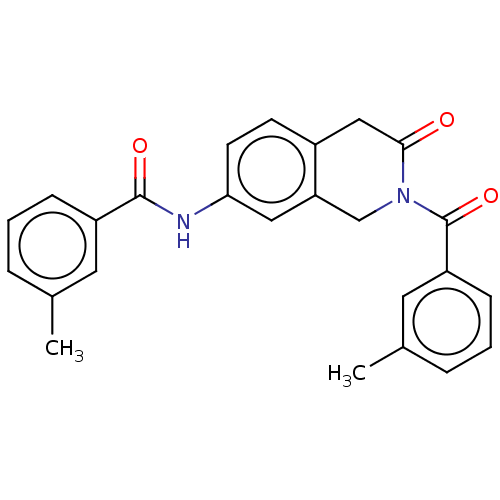



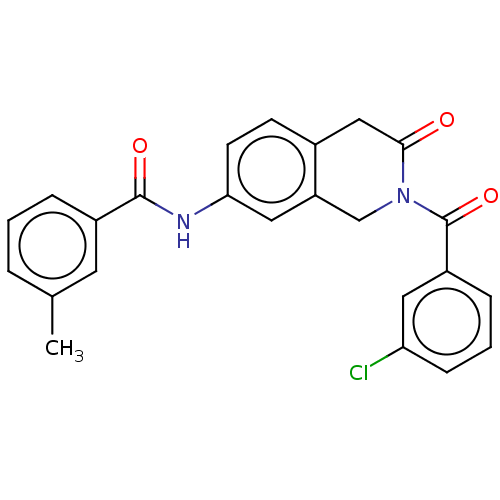
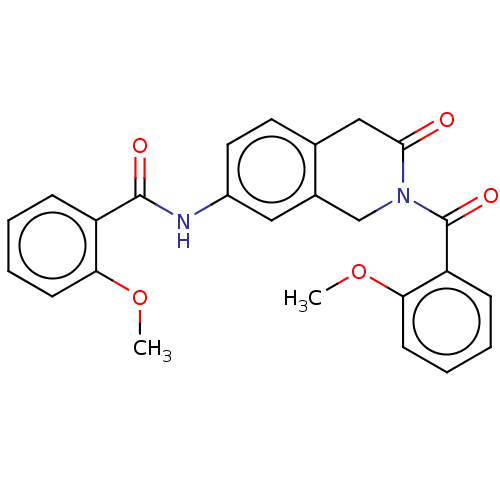


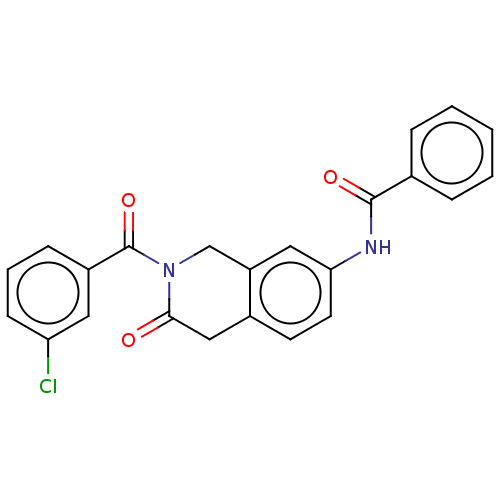


 3D Structure (crystal)
3D Structure (crystal)