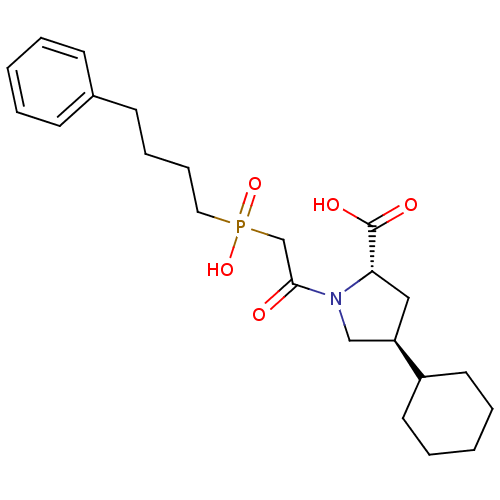
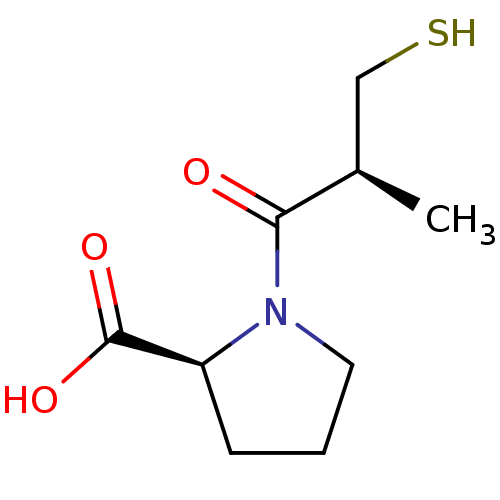
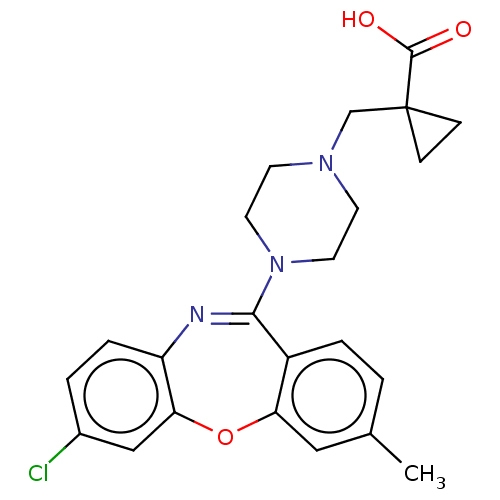
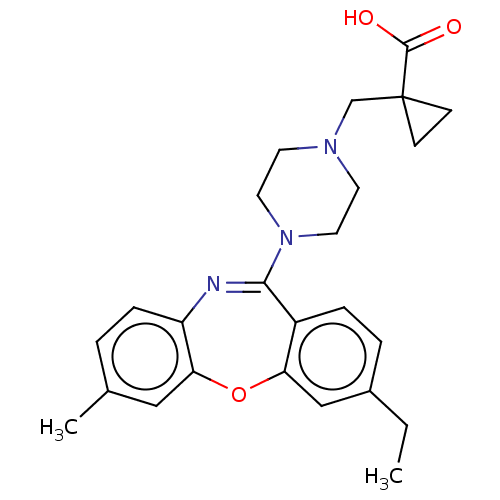
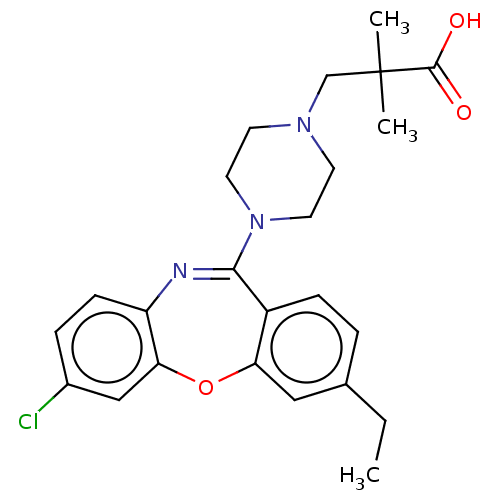
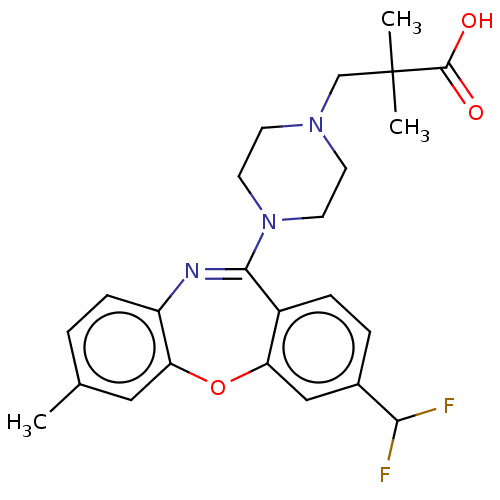
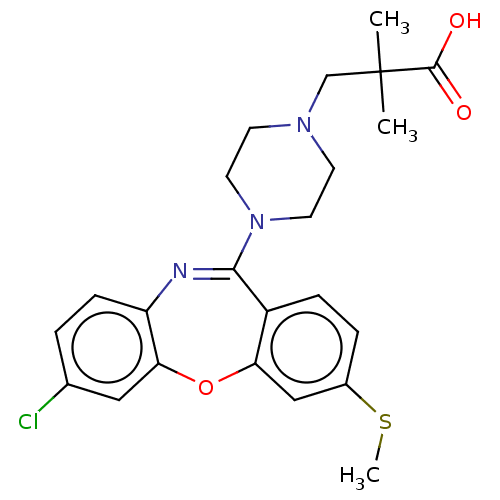
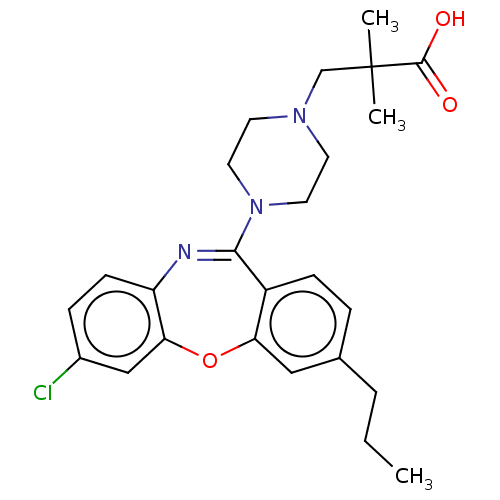
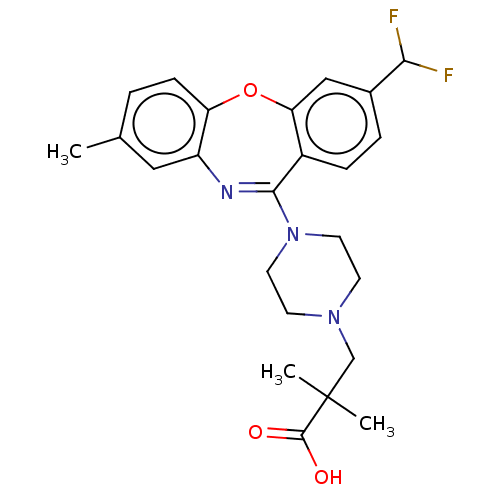
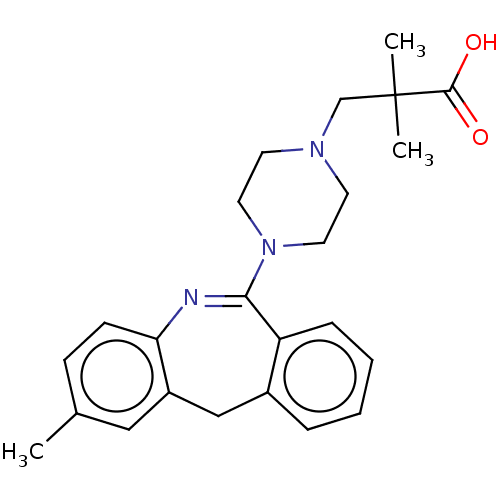
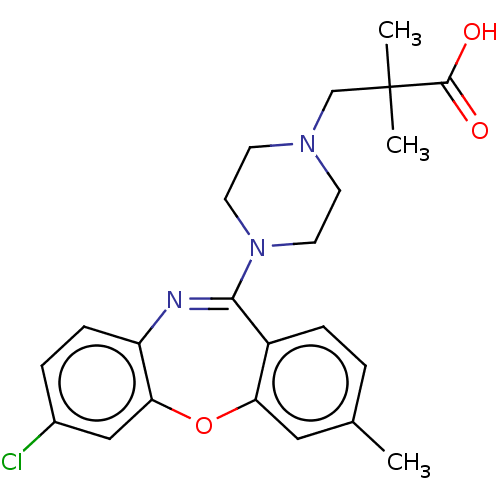
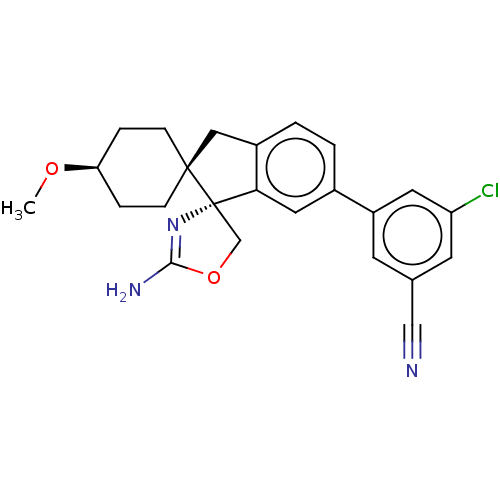
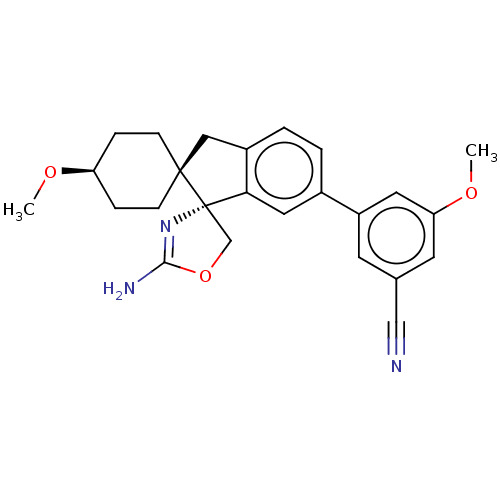
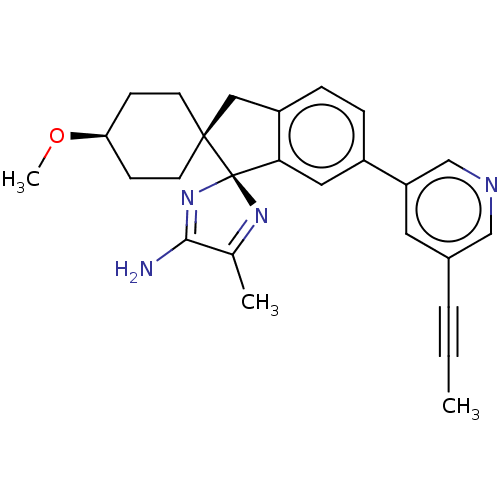
TargetAngiotensin-converting enzyme(Oryctolagus cuniculus)
Squibb Institute For Medical Research
Curated by ChEMBL
Squibb Institute For Medical Research
Curated by ChEMBL
Affinity DataKi: 0.400nMAssay Description:Inhibitory constant against rabbit lung Angiotensin I converting enzymeMore data for this Ligand-Target Pair
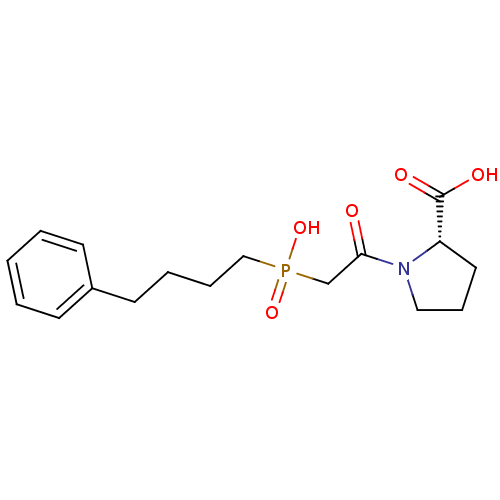
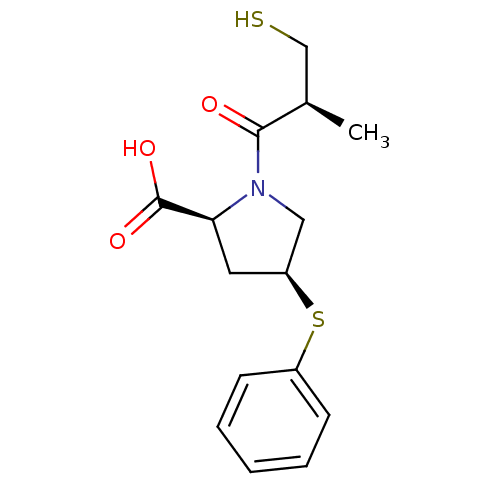
TargetCannabinoid receptor 2(Homo sapiens (Human))
University Of California Berkeley
Curated by ChEMBL
University Of California Berkeley
Curated by ChEMBL
Affinity DataKi: 0.900nMAssay Description:Displacement of [3H]-CP55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysisMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Oryctolagus cuniculus)
Squibb Institute For Medical Research
Curated by ChEMBL
Squibb Institute For Medical Research
Curated by ChEMBL
Affinity DataKi: 1.40nMAssay Description:Inhibitory activity against rabbit lung angiotensin-1 converting enzymeMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Oryctolagus cuniculus)
Squibb Institute For Medical Research
Curated by ChEMBL
Squibb Institute For Medical Research
Curated by ChEMBL
Affinity DataKi: 1.5nMAssay Description:Inhibitory activity against rabbit lung angiotensin-1 converting enzymeMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Oryctolagus cuniculus)
Squibb Institute For Medical Research
Curated by ChEMBL
Squibb Institute For Medical Research
Curated by ChEMBL
Affinity DataKi: 1.70nMAssay Description:Inhibitory activity against rabbit lung angiotensin-1 converting enzymeMore data for this Ligand-Target Pair
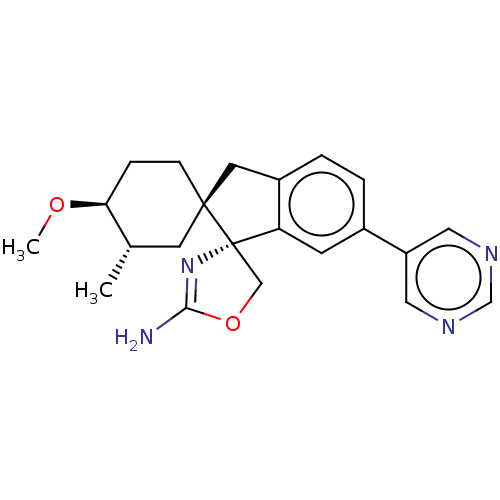
TargetCannabinoid receptor 1(Homo sapiens (Human))
University Of California Berkeley
Curated by ChEMBL
University Of California Berkeley
Curated by ChEMBL
Affinity DataKi: 8.80nMAssay Description:Displacement of [3H]-CP55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:1 μL of a compound of the present disclosure the reference compound (Table J) was transferred to an assay plate. 1 μL of 0.2 mM SB-206533 w...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:1 μL of a compound of the present disclosure the reference compound (Table J) was transferred to an assay plate. 1 μL of 0.2 mM SB-206533 w...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:1 μL of a compound of the present disclosure the reference compound (Table J) was transferred to an assay plate. 1 μL of 0.2 mM SB-206533 w...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:1 μL of a compound of the present disclosure the reference compound (Table J) was transferred to an assay plate. 1 μL of 0.2 mM SB-206533 w...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:1 μL of a compound of the present disclosure the reference compound (Table J) was transferred to an assay plate. 1 μL of 0.2 mM SB-206533 w...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:1 μL of a compound of the present disclosure the reference compound (Table J) was transferred to an assay plate. 1 μL of 0.2 mM SB-206533 w...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Inhibition of human BACE2 (1 to 473 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Inhibition of human BACE2 (1 to 473 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Inhibition of human BACE2 (1 to 473 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a...More data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Oryctolagus cuniculus)
Squibb Institute For Medical Research
Curated by ChEMBL
Squibb Institute For Medical Research
Curated by ChEMBL
Affinity DataKi: 15nMAssay Description:Inhibitory activity against rabbit lung angiotensin-1 converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Inhibition of human BACE1 (1 to 460 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a...More data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Inhibition of human BACE1 (1 to 460 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a...More data for this Ligand-Target Pair
Affinity DataKi: 21nMAssay Description:Inhibition of human BACE2 (1 to 473 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a...More data for this Ligand-Target Pair
Affinity DataKi: 23nMAssay Description:Inhibition of human BACE1 (1 to 460 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a...More data for this Ligand-Target Pair
Affinity DataKi: 23nMAssay Description:Inhibition of human BACE1 (1 to 460 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a...More data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:Inhibition of human BACE1 (1 to 460 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a...More data for this Ligand-Target Pair
Affinity DataKi: 26nMAssay Description:Inhibition of recombinant human BACE1 (1 to 460 residues) using CEVNLDAEFK as substrate preincubated for 10 mins followed by substrate addition measu...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 29nMAssay Description:Binding affinity to human ERG potassium channelMore data for this Ligand-Target Pair
Affinity DataKi: 37.5nMAssay Description:1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta...More data for this Ligand-Target Pair
Affinity DataKi: 37.5nMAssay Description:1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta...More data for this Ligand-Target Pair
Affinity DataKi: 37.5nMAssay Description:1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta...More data for this Ligand-Target Pair
Affinity DataKi: 37.5nMAssay Description:1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta...More data for this Ligand-Target Pair

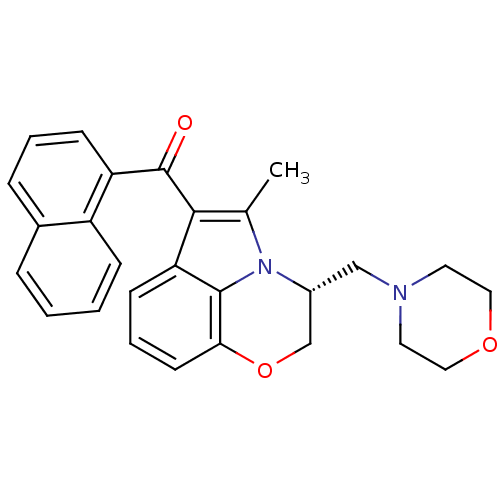
 3D Structure (crystal)
3D Structure (crystal)