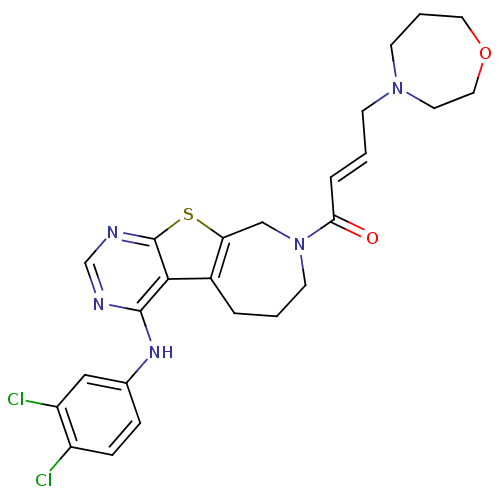
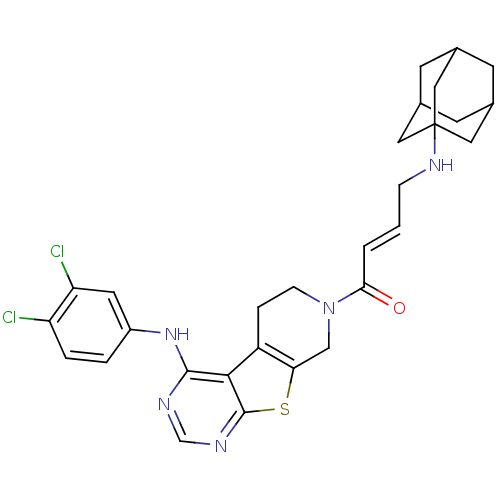
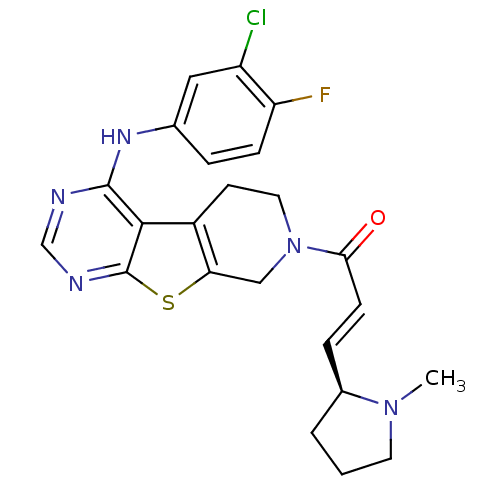
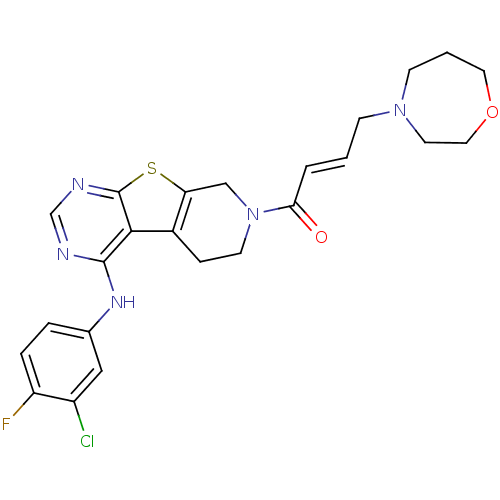
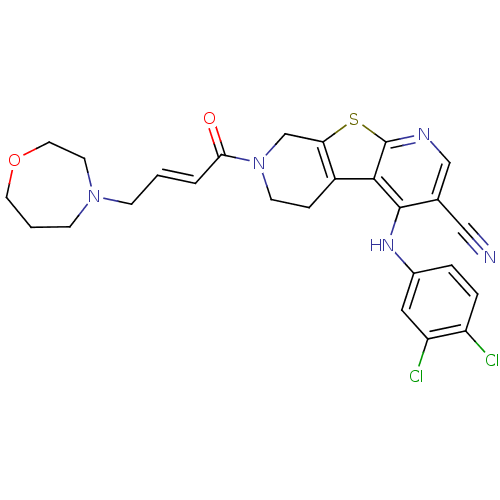
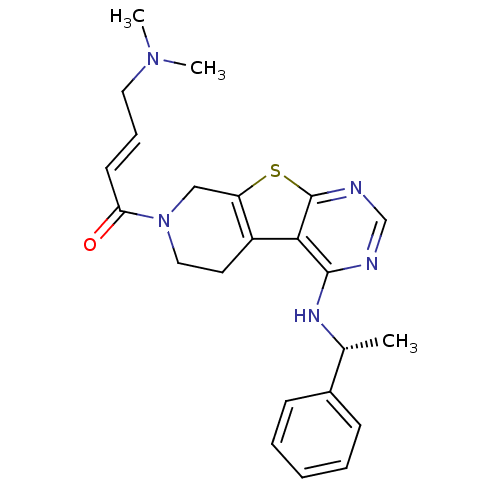
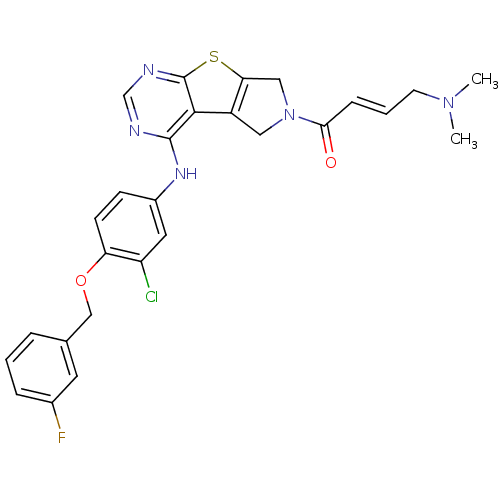
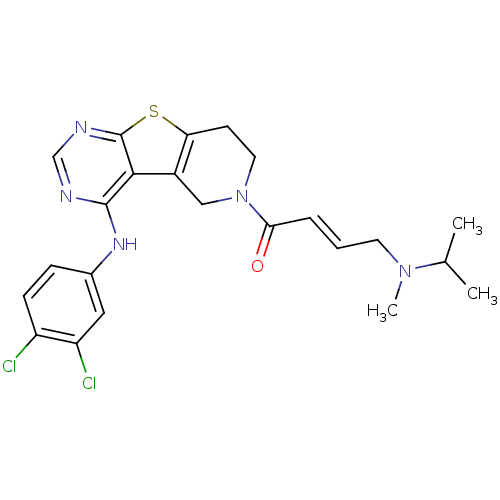
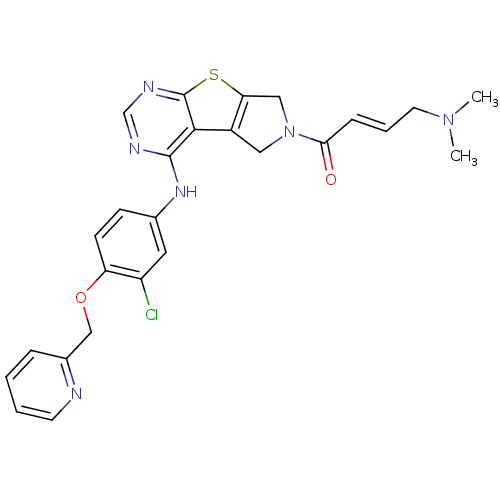
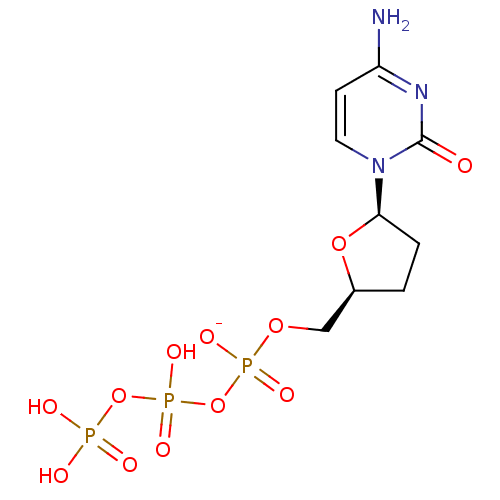
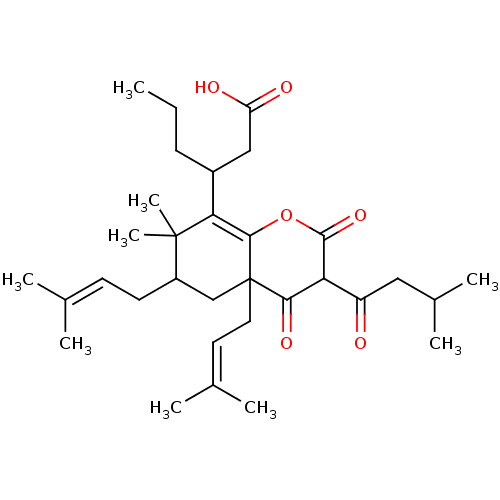
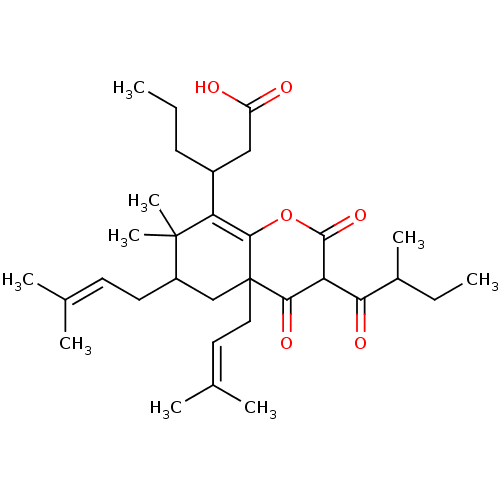
Affinity DataIC50: 0.00250nMAssay Description:In vitro antagonist potency in transactivation assay in neuroblastoma cells expressing human PR-B progesterone receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.00360nMAssay Description:In vitro antagonist potency in transactivation assay in neuroblastoma cells expressing human PR-A progesterone receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0250nMAssay Description:In vitro antagonist potency in transactivation assay in neuroblastoma cells expressing human PR-B progesterone receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0280nMAssay Description:In vitro antgonist potency in transactivation assay in neuroblastoma cells expressing human PR-A progesterone receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.0 T: 2°CAssay Description:EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay.More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.0 T: 2°CAssay Description:EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay.More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.0 T: 2°CAssay Description:EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay.More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.0 T: 2°CAssay Description:EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay.More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.0 T: 2°CAssay Description:EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay.More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.0 T: 2°CAssay Description:EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay.More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.0 T: 2°CAssay Description:EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay.More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.0 T: 2°CAssay Description:EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay.More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.0 T: 2°CAssay Description:EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay.More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.0 T: 2°CAssay Description:EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay.More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMpH: 7.0 T: 2°CAssay Description:EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay.More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMpH: 7.0 T: 2°CAssay Description:EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay.More data for this Ligand-Target Pair
Affinity DataIC50: 2nMpH: 7.0 T: 2°CAssay Description:EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay.More data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:In vitro antagonist potency in transactivation assay in NIH3T3 cells expressing glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 4.30nMpH: 7.0 T: 2°CAssay Description:EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay.More data for this Ligand-Target Pair
Affinity DataIC50: 4.60nMpH: 7.0 T: 2°CAssay Description:EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay.More data for this Ligand-Target Pair
Affinity DataIC50: 5nMpH: 7.0 T: 2°CAssay Description:EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay.More data for this Ligand-Target Pair
Affinity DataIC50: 6.40nMpH: 7.0 T: 2°CAssay Description:EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay.More data for this Ligand-Target Pair
Affinity DataIC50: 9.20nMpH: 7.0 T: 2°CAssay Description:EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay.More data for this Ligand-Target Pair
Affinity DataIC50: 10nMpH: 7.0 T: 2°CAssay Description:EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay.More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:In vitro antagonist potency in transactivation assay in CV-1 cells expressing androgen receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMpH: 7.0 T: 2°CAssay Description:EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay.More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:In vitro antagonist potency in transactivation assay in NIH3T3 cells expressing glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMpH: 7.0 T: 2°CAssay Description:EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay.More data for this Ligand-Target Pair
Affinity DataIC50: 54nMAssay Description:In vitro antagonist potency in transactivation assay in CV-1 cells expressing androgen receptorMore data for this Ligand-Target Pair
TargetDNA polymerase beta(Homo sapiens (Human))
National Center For Scientific Research (Cnrs)-Pierre Fabre
Curated by ChEMBL
National Center For Scientific Research (Cnrs)-Pierre Fabre
Curated by ChEMBL
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of human recombinant DNA polymerase beta assessed as fluorescein-12-dCTP incorporation by fluorimetryMore data for this Ligand-Target Pair
TargetDNA polymerase beta(Homo sapiens (Human))
National Center For Scientific Research (Cnrs)-Pierre Fabre
Curated by ChEMBL
National Center For Scientific Research (Cnrs)-Pierre Fabre
Curated by ChEMBL
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of human recombinant DNA polymerase beta assessed as fluorescein-12-dCTP incorporation by fluorimetryMore data for this Ligand-Target Pair
TargetDNA polymerase beta(Homo sapiens (Human))
National Center For Scientific Research (Cnrs)-Pierre Fabre
Curated by ChEMBL
National Center For Scientific Research (Cnrs)-Pierre Fabre
Curated by ChEMBL
Affinity DataIC50: 2.80E+4nMAssay Description:Inhibition of human recombinant DNA polymerase beta assessed as fluorescein-12-dCTP incorporation by fluorimetryMore data for this Ligand-Target Pair
TargetDNA polymerase beta(Homo sapiens (Human))
National Center For Scientific Research (Cnrs)-Pierre Fabre
Curated by ChEMBL
National Center For Scientific Research (Cnrs)-Pierre Fabre
Curated by ChEMBL
Affinity DataIC50: 7.30E+4nMAssay Description:Inhibition of human recombinant DNA polymerase beta assessed as fluorescein-12-dCTP incorporation by fluorimetryMore data for this Ligand-Target Pair
TargetDNA polymerase beta(Homo sapiens (Human))
National Center For Scientific Research (Cnrs)-Pierre Fabre
Curated by ChEMBL
National Center For Scientific Research (Cnrs)-Pierre Fabre
Curated by ChEMBL
Affinity DataIC50: 3.42E+5nMAssay Description:Inhibition of human recombinant DNA polymerase beta assessed as fluorescein-12-dCTP incorporation by fluorimetryMore data for this Ligand-Target Pair
TargetAryl hydrocarbon receptor(Homo sapiens (Human))
Max Planck Institute Of Molecular Physiology
Curated by ChEMBL
Max Planck Institute Of Molecular Physiology
Curated by ChEMBL
Affinity DataEC50: 3.20nMAssay Description:Activation of AhR in human HepG2 cells assessed as induction of XRE-dependent reporter activity incubated for 4 hrs by Renilla/Firefly based dual-luc...More data for this Ligand-Target Pair
TargetAryl hydrocarbon receptor(Mus musculus (Mouse))
Max Planck Institute Of Molecular Physiology
Curated by ChEMBL
Max Planck Institute Of Molecular Physiology
Curated by ChEMBL
Affinity DataEC50: 0.0580nMAssay Description:Activation of AhR in mouse NIH3T3 cells assessed as induction of XRE-dependent reporter activity incubated for 4 hrs by Renilla/Firefly based dual-lu...More data for this Ligand-Target Pair
TargetAryl hydrocarbon receptor(Homo sapiens (Human))
Max Planck Institute Of Molecular Physiology
Curated by ChEMBL
Max Planck Institute Of Molecular Physiology
Curated by ChEMBL
Affinity DataEC50: 0.700nMAssay Description:Activation of AhR in human HaCaT cells assessed as induction of XRE-dependent reporter activity incubated for 4 hrs by Renilla/Firefly based dual-luc...More data for this Ligand-Target Pair
TargetAryl hydrocarbon receptor(Mus musculus (Mouse))
Max Planck Institute Of Molecular Physiology
Curated by ChEMBL
Max Planck Institute Of Molecular Physiology
Curated by ChEMBL
Affinity DataEC50: 71nMAssay Description:Activation of AhR in mouse Hepa1c1c7 cells assessed as induction of XRE-dependent reporter activity incubated for 4 hrs by Renilla/Firefly based dual...More data for this Ligand-Target Pair



 3D Structure (crystal)
3D Structure (crystal)