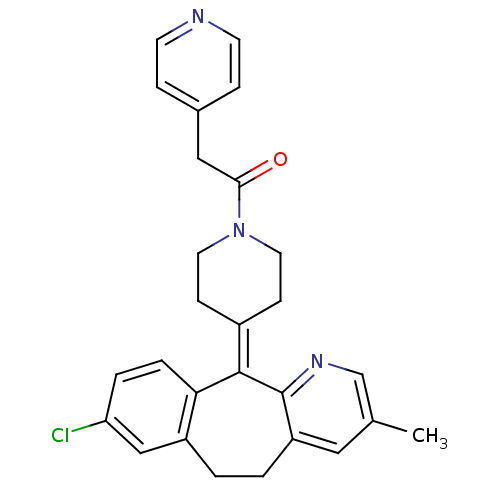
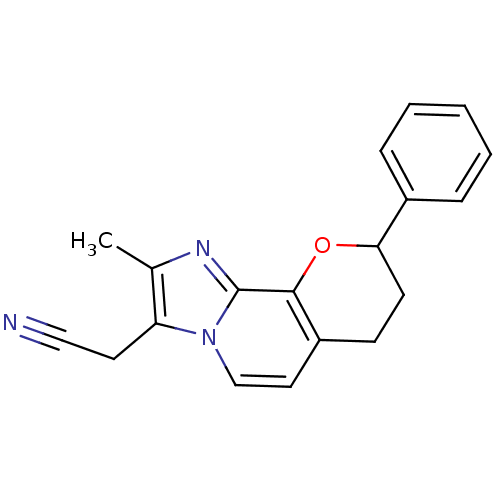
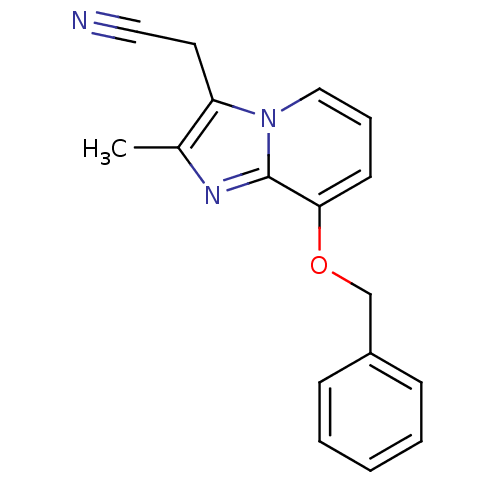
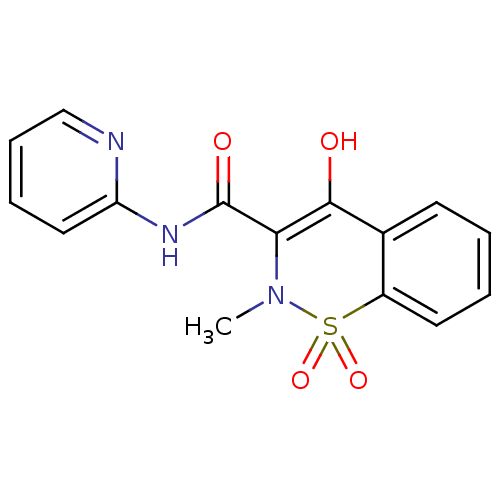
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 0.0100nMAssay Description:Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 0.140nMAssay Description:Antagonistic activity of the compound against muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 0.200nMAssay Description:Antagonistic activity of the compound against muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 0.230nMAssay Description:Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 0.290nMAssay Description:Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 0.510nMAssay Description:Antagonistic activity of the compound against muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 0.510nMAssay Description:Antagonistic activity of the compound against muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 0.510nMAssay Description:Binding affinity for human Muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 0.810nMAssay Description:Antagonistic activity of the compound against muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 0.890nMAssay Description:Binding affinity for human Muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 0.900nMAssay Description:Binding affinity for human Muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 0.990nMAssay Description:Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 1.20nMAssay Description:Antagonistic activity of the compound against muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 1.40nMAssay Description:Antagonistic activity of the compound against muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 2.40nMAssay Description:Binding affinity for human Muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 2.70nMAssay Description:Binding affinity for human Muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 2.70nMAssay Description:Binding affinity for human Muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
Affinity DataKi: 5.5nMAssay Description:Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranesMore data for this Ligand-Target Pair
Affinity DataKi: 6.40nMAssay Description:Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranesMore data for this Ligand-Target Pair
Affinity DataKi: 6.5nMAssay Description:Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranesMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 6.60nMAssay Description:Binding affinity for human Muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 9.60nMAssay Description:Binding affinity for human Muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 12nMAssay Description:Binding affinity for human Muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 12nMAssay Description:Binding affinity for human Muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 32nMAssay Description:Binding affinity for human Muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 33nMAssay Description:Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
Affinity DataKi: 49nMAssay Description:Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranesMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 104nMAssay Description:Binding affinity for human Muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
Affinity DataKi: 140nMAssay Description:Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranesMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 221nMAssay Description:Binding affinity for human Muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
Affinity DataKi: 290nMAssay Description:Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranesMore data for this Ligand-Target Pair
Affinity DataKi: 320nMAssay Description:Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranesMore data for this Ligand-Target Pair
Affinity DataKi: 970nMAssay Description:Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranesMore data for this Ligand-Target Pair
Affinity DataKi: 2.50E+3nMAssay Description:Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranesMore data for this Ligand-Target Pair
Affinity DataKi: 3.60E+3nMAssay Description:Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranesMore data for this Ligand-Target Pair
Affinity DataKi: 3.60E+3nMAssay Description:Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranesMore data for this Ligand-Target Pair
Affinity DataKi: 3.60E+3nMAssay Description:Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranesMore data for this Ligand-Target Pair
Affinity DataKi: 3.70E+3nMAssay Description:Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranesMore data for this Ligand-Target Pair
Affinity DataKi: 5.40E+3nMAssay Description:Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranesMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranesMore data for this Ligand-Target Pair
TargetDimer of Protein farnesyltransferase subunit beta(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 14nMAssay Description:Inhibitory activity against recombinant human farnesyltransferaseMore data for this Ligand-Target Pair
TargetDimer of Protein farnesyltransferase subunit beta(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 14nMAssay Description:Inhibitory activity against recombinant human farnesyltransferaseMore data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:In vitro inhibitory activity against Prostaglandin G/H synthase in rat neutrophilsMore data for this Ligand-Target Pair
TargetDimer of Protein farnesyltransferase subunit beta(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 60nMAssay Description:Inhibitory activity against recombinant human farnesyltransferaseMore data for this Ligand-Target Pair
TargetPotassium-transporting ATPase alpha chain 1/subunit beta(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 64nMAssay Description:Inhibition of H+/K+ ATPase as reduced acid formation in rabbit gastric glandsMore data for this Ligand-Target Pair
TargetPotassium-transporting ATPase alpha chain 1/subunit beta(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 65nMAssay Description:Inhibition of H+/K+ ATPase as reduced acid formation in rabbit gastric glandsMore data for this Ligand-Target Pair
TargetPotassium-transporting ATPase alpha chain 1/subunit beta(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 92nMpH: 7.4Assay Description:Inhibition of purified H+/K+ ATPase at pH 7.4 as released inorganic phosphate from ATP using hog stomach gastric membrane vesiclesMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:In vitro inhibitory activity against Prostaglandin G/H synthase in rat neutrophilsMore data for this Ligand-Target Pair
TargetPotassium-transporting ATPase alpha chain 1/subunit beta(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 130nMAssay Description:Inhibition of H+/K+ ATPase as reduced acid formation in rabbit gastric glandsMore data for this Ligand-Target Pair
TargetDimer of Protein farnesyltransferase subunit beta(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 160nMAssay Description:Inhibitory activity against recombinant human farnesyltransferaseMore data for this Ligand-Target Pair

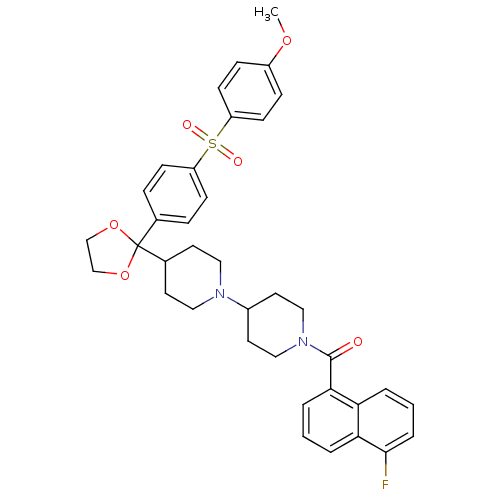
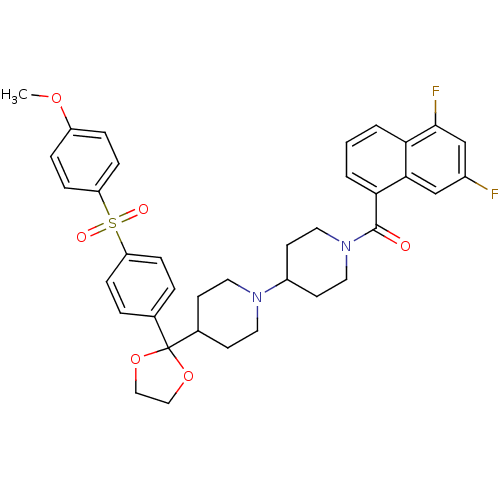

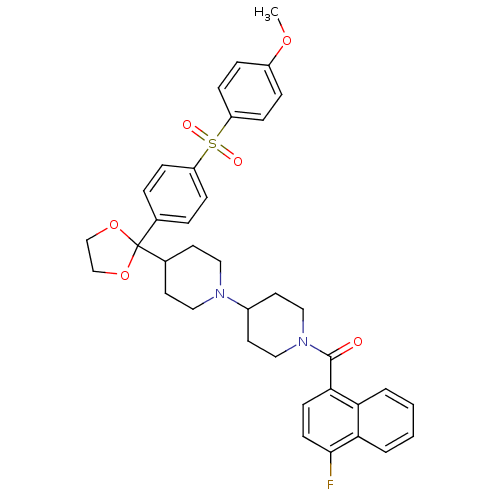
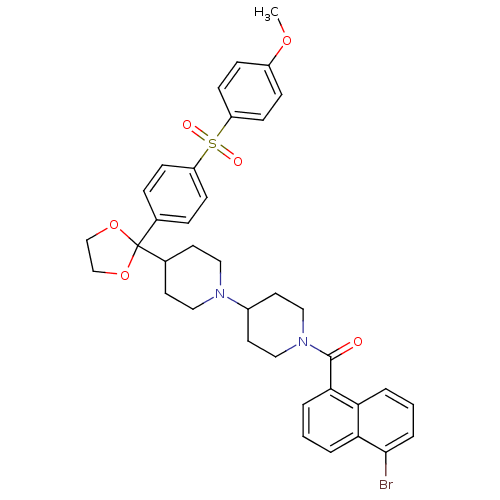

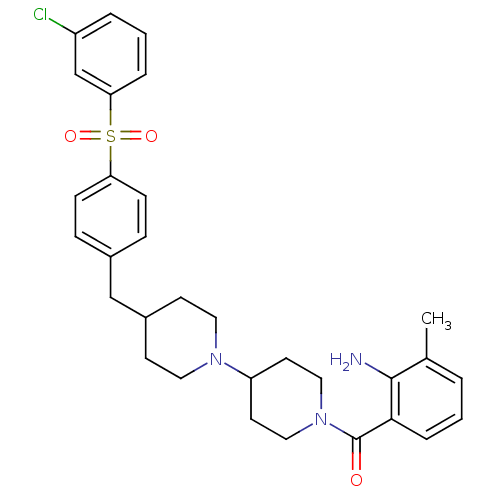


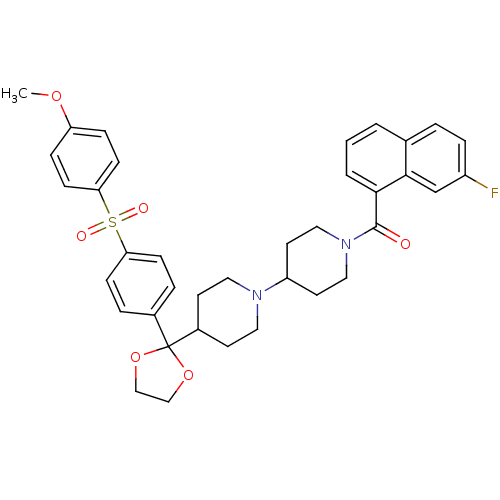


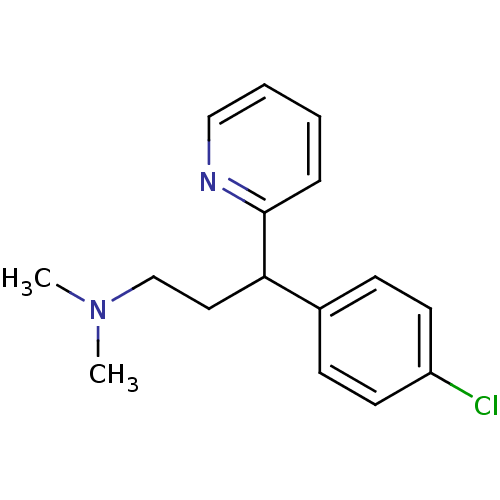

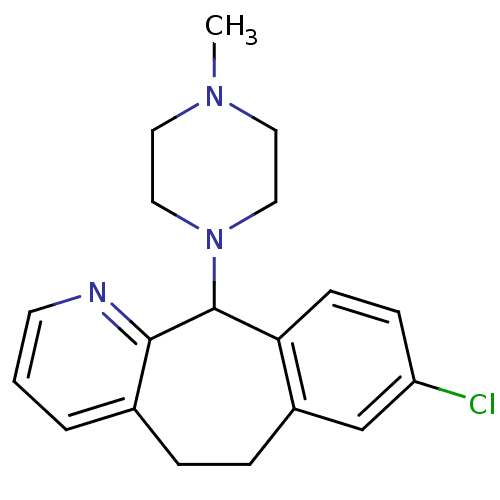




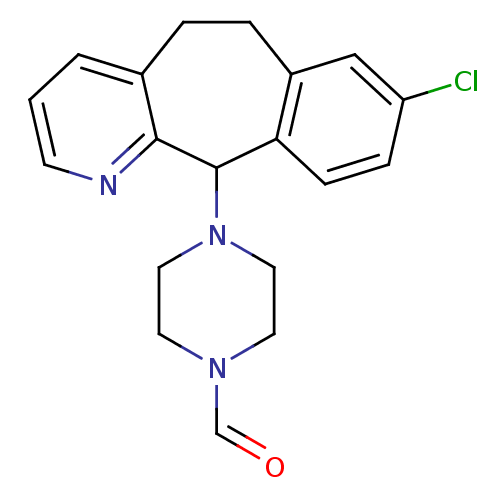



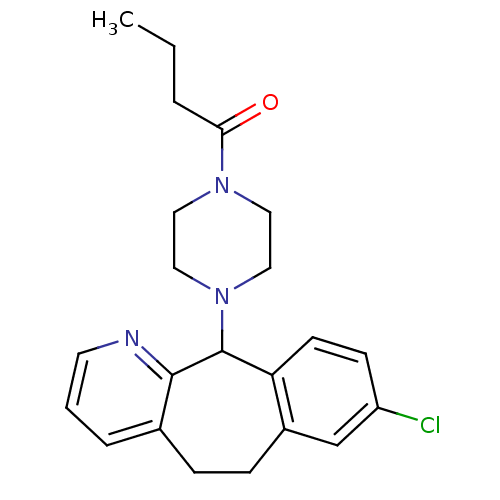

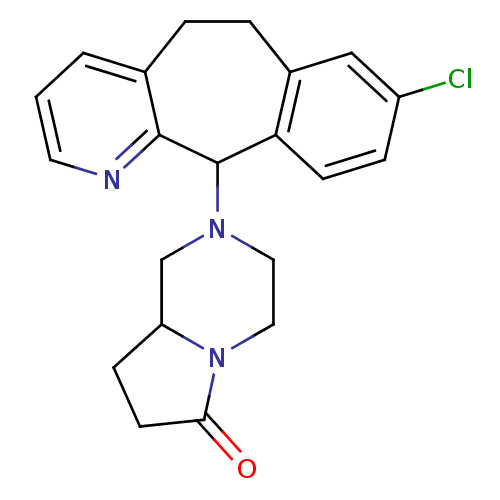


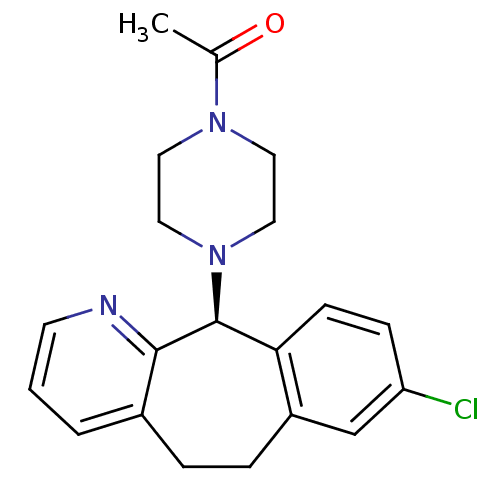
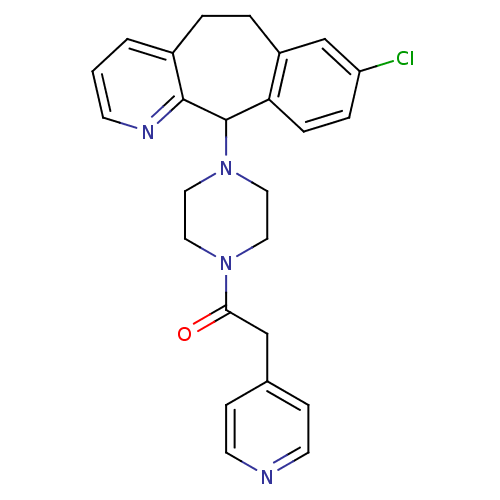
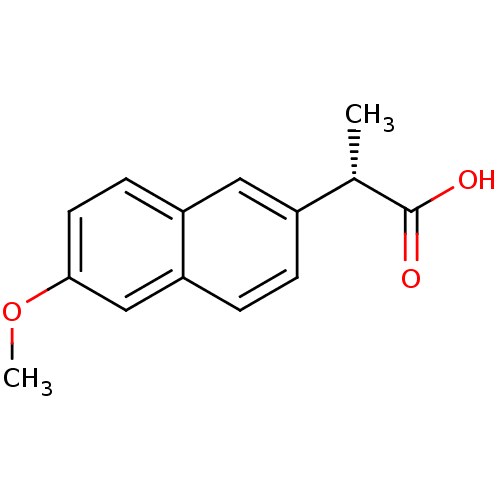
 3D Structure (crystal)
3D Structure (crystal)