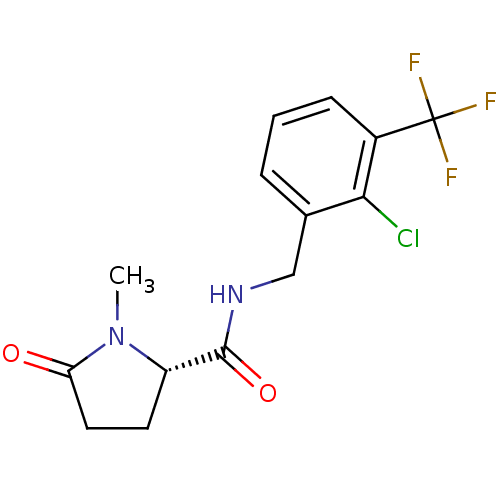
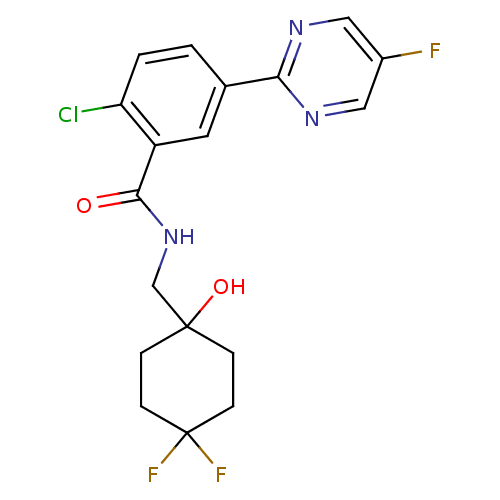
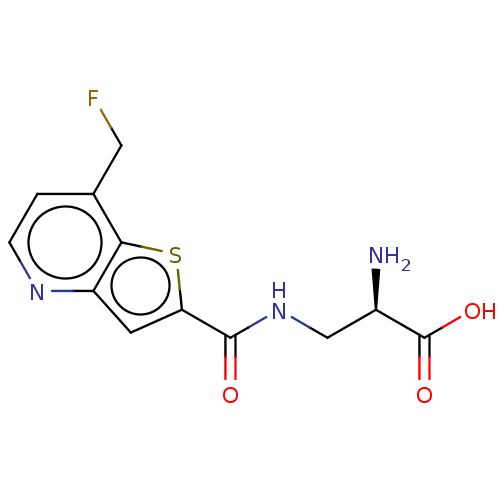
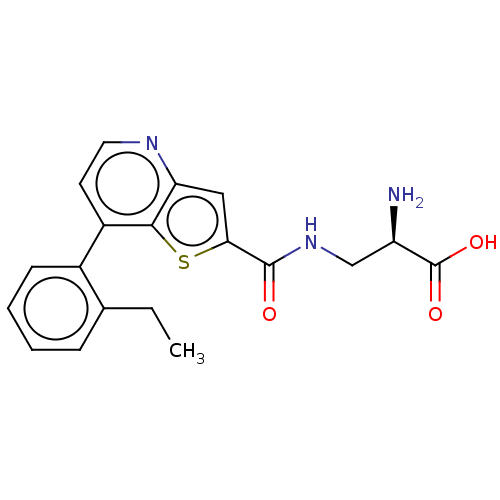
Affinity DataKi: 3.90nMAssay Description:Displacement of [3H]-A-804598 from rat P2X7 receptor expressed in HEK293 cell membrane by in vitro binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 5.60nMAssay Description:Displacement of [3H]-A-804598 from human P2X7 receptor expressed in HEK293 cell membrane by in vitro binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 5.70nMAssay Description:Displacement of [3H]-A-804598 from human P2X7 receptor expressed in HEK293 cell membrane by in vitro binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 7.40nMAssay Description:Displacement of [3H]-A-804598 from human P2X7 receptor expressed in HEK293 cell membrane by in vitro binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 8.90nMAssay Description:Displacement of [3H]-A-804598 from human P2X7 receptor expressed in HEK293 cell membrane by in vitro binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Displacement of [3H]-A-804598 from rat P2X7 receptor expressed in HEK293 cell membrane by in vitro binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Displacement of [3H]-A-804598 from human P2X7 receptor expressed in HEK293 cell membrane by in vitro binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Displacement of [3H]-A-804598 from rat P2X7 receptor expressed in HEK293 cell membrane by in vitro binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 48nMAssay Description:Displacement of [3H]-A-804598 from mouse P2X7 receptor expressed in HEK293 cell membrane by in vitro binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 53nMAssay Description:Displacement of [3H]-A-804598 from human P2X7 receptor expressed in HEK293 cell membrane by in vitro binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 55nMAssay Description:Displacement of [3H]-A-804598 from human P2X7 receptor expressed in HEK293 cell membrane by in vitro binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 59nMAssay Description:Displacement of [3H]-A-804598 from rat P2X7 receptor expressed in HEK293 cell membrane by in vitro binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 60nMAssay Description:Displacement of [3H]-A-804598 from mouse P2X7 receptor expressed in HEK293 cell membrane by in vitro binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 63nMAssay Description:To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ...More data for this Ligand-Target Pair
Affinity DataKi: 63nMAssay Description:To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ...More data for this Ligand-Target Pair
Affinity DataKi: 68nMAssay Description:Displacement of [3H]-A-804598 from human P2X7 receptor expressed in HEK293 cell membrane by in vitro binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 96nMAssay Description:To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ...More data for this Ligand-Target Pair
Affinity DataKi: 96nMAssay Description:To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ...More data for this Ligand-Target Pair
Affinity DataKi: 98nMAssay Description:Displacement of [3H]-A-804598 from rat P2X7 receptor expressed in HEK293 cell membrane by in vitro binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Displacement of [3H]-A-804598 from mouse P2X7 receptor expressed in HEK293 cell membrane by in vitro binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 130nMAssay Description:Displacement of [3H]-A-804598 from human P2X7 receptor expressed in HEK293 cell membrane by in vitro binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 130nMAssay Description:Displacement of [3H]-A-804598 from rat P2X7 receptor expressed in HEK293 cell membrane by in vitro binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 140nMAssay Description:To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ...More data for this Ligand-Target Pair
Affinity DataKi: 140nMAssay Description:To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ...More data for this Ligand-Target Pair
Affinity DataKi: 170nMAssay Description:To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ...More data for this Ligand-Target Pair
Affinity DataKi: 170nMAssay Description:To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ...More data for this Ligand-Target Pair
Affinity DataKi: 180nMAssay Description:To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ...More data for this Ligand-Target Pair
Affinity DataKi: 180nMAssay Description:Displacement of [3H]-A-804598 from mouse P2X7 receptor expressed in HEK293 cell membrane by in vitro binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 180nMAssay Description:To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ...More data for this Ligand-Target Pair
Affinity DataKi: 200nMAssay Description:Displacement of [3H]-A-804598 from rat P2X7 receptor expressed in HEK293 cell membrane by in vitro binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 220nMAssay Description:To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ...More data for this Ligand-Target Pair
Affinity DataKi: 220nMAssay Description:To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ...More data for this Ligand-Target Pair
Affinity DataKi: 220nMAssay Description:To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ...More data for this Ligand-Target Pair
Affinity DataKi: 260nMAssay Description:To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ...More data for this Ligand-Target Pair
Affinity DataKi: 260nMAssay Description:To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ...More data for this Ligand-Target Pair
Affinity DataKi: 270nMAssay Description:To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ...More data for this Ligand-Target Pair
Affinity DataKi: 360nMAssay Description:To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ...More data for this Ligand-Target Pair
Affinity DataKi: 360nMAssay Description:To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ...More data for this Ligand-Target Pair
Affinity DataKi: 490nMAssay Description:To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ...More data for this Ligand-Target Pair
Affinity DataKi: 490nMAssay Description:To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ...More data for this Ligand-Target Pair
Affinity DataKi: 510nMAssay Description:Displacement of [3H]-A-804598 from rat P2X7 receptor expressed in HEK293 cell membrane by in vitro binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 690nMAssay Description:To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ...More data for this Ligand-Target Pair
Affinity DataKi: 690nMAssay Description:To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ...More data for this Ligand-Target Pair
Affinity DataKi: 860nMAssay Description:To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ...More data for this Ligand-Target Pair
Affinity DataKi: 860nMAssay Description:To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ...More data for this Ligand-Target Pair
Affinity DataKi: 870nMAssay Description:To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ...More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nMAssay Description:Displacement of [3H]-A-804598 from rat P2X7 receptor expressed in HEK293 cell membrane by in vitro binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 3.90E+3nMAssay Description:To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ...More data for this Ligand-Target Pair
Affinity DataKi: 3.90E+3nMAssay Description:To determine the affinity of the compounds of the present invention a SPA is used. The assay is run in a 384-plate format (OptiPlate-384) where each ...More data for this Ligand-Target Pair
Affinity DataKi: 4.80E+3nMAssay Description:Displacement of [3H]-A-804598 from rat P2X7 receptor expressed in HEK293 cell membrane by in vitro binding assayMore data for this Ligand-Target Pair