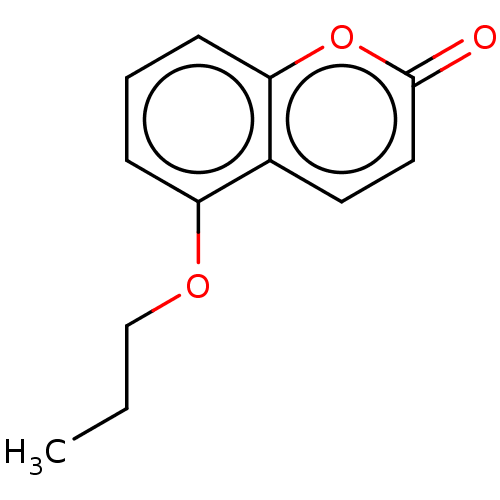
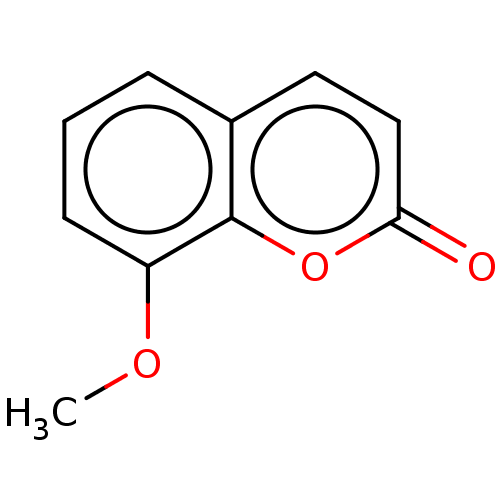
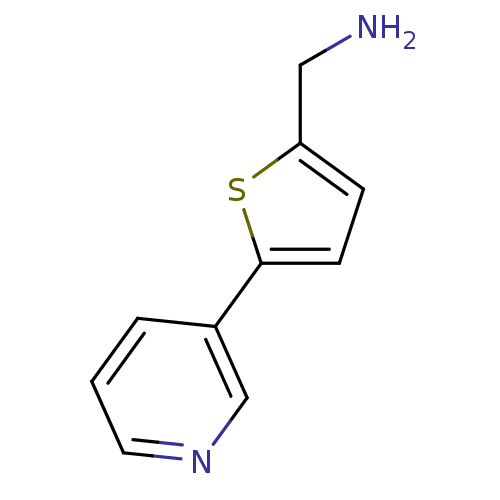
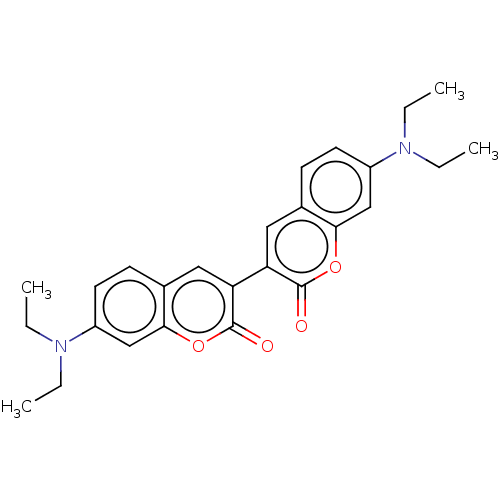
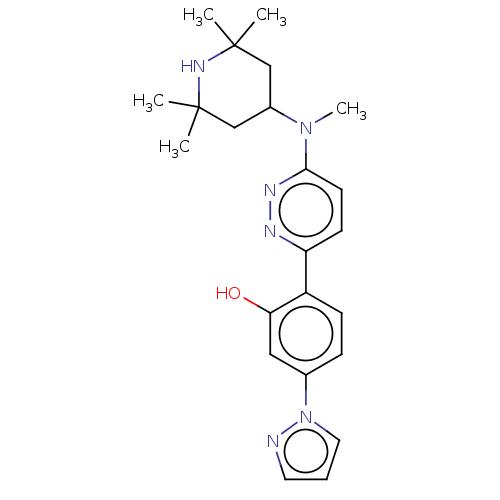
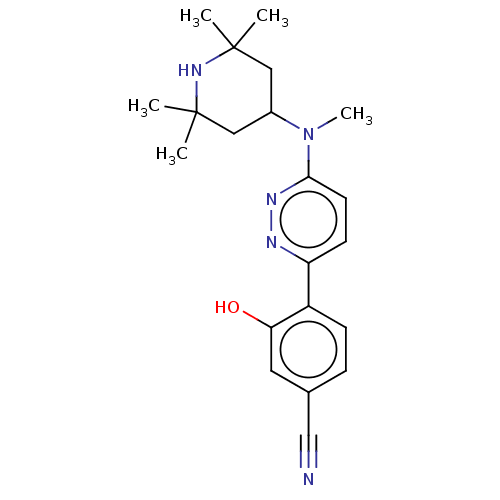
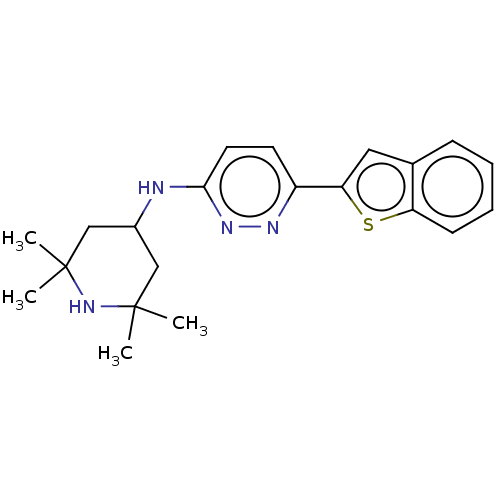
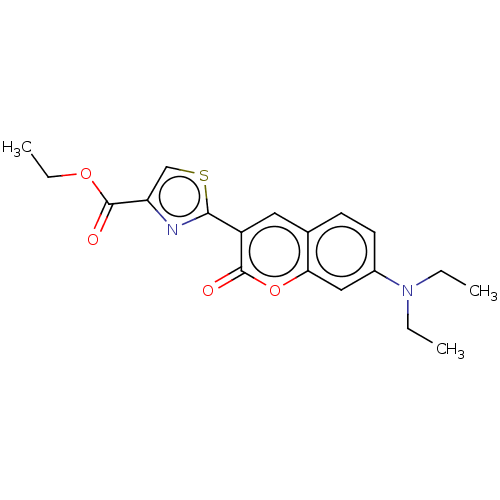
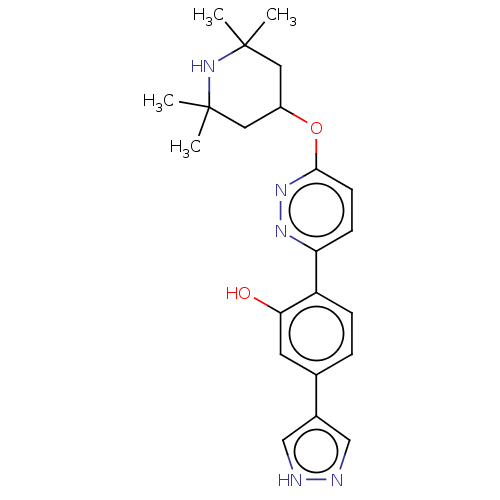
Affinity DataKi: 0.450nMAssay Description:Displacement of [3H]NMS from human M2R expressed in CHOK1 cells after 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.680nMAssay Description:Displacement of [3H]NMS from human M2R expressed in CHOK1 cells after 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.10nMAssay Description:Displacement of [3H]NMS from human M3R expressed in CHOK1 cells after 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.5nMAssay Description:Displacement of [3H]NMS from human M2R expressed in CHOK1 cells after 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.60nMAssay Description:Displacement of [3H]NMS from human M3R expressed in CHOK1 cells after 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Competitive inhibition of CYP2A6 (unknown origin)More data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 28nMAssay Description:Displacement of [3H]NMS from human M3R expressed in CHOK1 cells after 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Competitive inhibition of CYP2A6 (unknown origin)More data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 50nMAssay Description:Mixed type inhibition of CYP2A6 (unknown origin)More data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 80nMAssay Description:Competitive inhibition of CYP2A6 (unknown origin)More data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 90nMAssay Description:Mixed type inhibition of CYP2A6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 250nMAssay Description:Competitive inhibition of CYP2A6 (unknown origin)More data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 260nMAssay Description:Non competitive inhibition of CYP2A6 in human liver microsome using coumarin as substrate preincubated with NADPH for 5 mins and measured after 30 mi...More data for this Ligand-Target Pair
Affinity DataKi: 540nMAssay Description:Competitive inhibition of CYP2A6 (unknown origin)More data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 2.20E+3nMAssay Description:Competitive inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 4.00E+3nMAssay Description:Competitive inhibition of CYP2A6 (unknown origin)More data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 2.50E+4nMAssay Description:Non competitive inhibition of CYP3A4 in human liver microsome using coumarin as substrate preincubated with NADPH for 5 mins and measured after 30 mi...More data for this Ligand-Target Pair
Affinity DataKi: 2.81E+4nMAssay Description:Mixed type inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 2.94E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 4.66E+4nMAssay Description:Mixed type inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 4.24E+5nMAssay Description:Non competitive inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 4.48E+5nMAssay Description:Non competitive inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 29nMAssay Description:Inhibition of CYP19 in human placental microsome using [1beta-3H]-androstenedione as substrate after 15 mins in presence of NADPH by liquid scintilla...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of CYP19 in human placental microsome using [1beta-3H]-androstenedione as substrate after 15 mins in presence of NADPH by liquid scintilla...More data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of CYP2A6 (unknown origin)More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 43nMAssay Description:Inhibition of purified HIV-1 reverse transcriptaseMore data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of CYP2A6 (unknown origin)More data for this Ligand-Target Pair
Ligand Info
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 80nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta countingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 110nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta countingMore data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:Inhibition of CYP2A6 (unknown origin)More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 170nMAssay Description:Inhibition of CYP2A6 (unknown origin)More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 200nMAssay Description:Inhibition of CYP2A6 (unknown origin)More data for this Ligand-Target Pair
Ligand Info
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 230nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta countingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 270nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta countingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta countingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta countingMore data for this Ligand-Target Pair
Affinity DataIC50: 460nMAssay Description:Inhibition of CYP2A6 (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 600nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta countingMore data for this Ligand-Target Pair
Affinity DataIC50: 1.06E+3nMAssay Description:Inhibition of CYP2A6 (unknown origin)More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of CYP19 in human placental microsome using [1beta-3H]-androstenedione as substrate after 15 mins in presence of NADPH by liquid scintilla...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of CYP19 in human placental microsome using [1beta-3H]-androstenedione as substrate after 15 mins in presence of NADPH by liquid scintilla...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta countingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.20E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta countingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.50E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta countingMore data for this Ligand-Target Pair
Affinity DataIC50: 2.60E+3nMAssay Description:Inhibition of CYP19 in human placental microsome using [1beta-3H]-androstenedione as substrate after 15 mins in presence of NADPH by liquid scintilla...More data for this Ligand-Target Pair
Affinity DataIC50: 3.10E+3nMAssay Description:Inhibition of CYP19 in human placental microsome using [1beta-3H]-androstenedione as substrate after 15 mins in presence of NADPH by liquid scintilla...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4.50E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta countingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 6.00E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta countingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 6.00E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta countingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 6.30E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta countingMore data for this Ligand-Target Pair

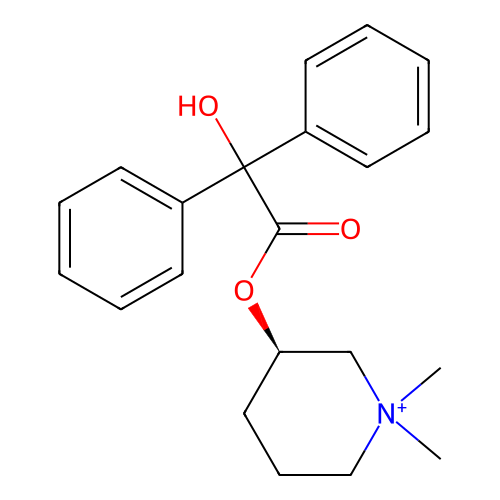
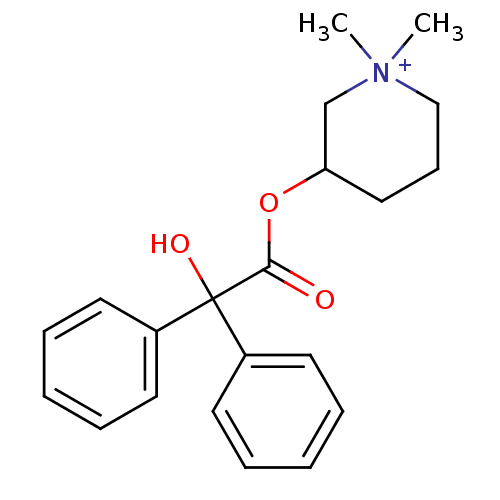
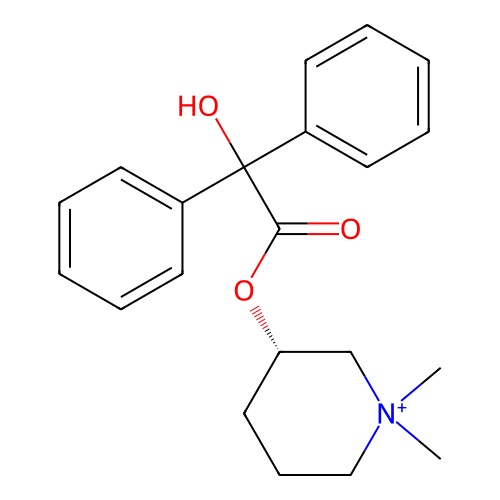




 3D Structure (crystal)
3D Structure (crystal)