Target72 kDa type IV collagenase(Homo sapiens (Human))
Preclinical Research Novartis Pharma
Curated by ChEMBL
Preclinical Research Novartis Pharma
Curated by ChEMBL
Affinity DataKi: 0.300nMAssay Description:Inhibitory potency against Matrix metalloprotease-2 (MMP-2)More data for this Ligand-Target Pair
Target72 kDa type IV collagenase(Homo sapiens (Human))
Preclinical Research Novartis Pharma
Curated by ChEMBL
Preclinical Research Novartis Pharma
Curated by ChEMBL
Affinity DataKi: 0.300nMAssay Description:Inhibitory potency against Matrix metalloprotease-2 (MMP-2)More data for this Ligand-Target Pair
Target72 kDa type IV collagenase(Homo sapiens (Human))
Preclinical Research Novartis Pharma
Curated by ChEMBL
Preclinical Research Novartis Pharma
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:Inhibitory potency against Matrix metalloprotease-2 (MMP-2)More data for this Ligand-Target Pair
TargetInterstitial collagenase(Homo sapiens (Human))
Preclinical Research Novartis Pharma
Curated by ChEMBL
Preclinical Research Novartis Pharma
Curated by ChEMBL
Affinity DataKi: 0.600nMAssay Description:Inhibitory potency against Matrix metalloprotease-1 (MMP-1)More data for this Ligand-Target Pair
TargetDisintegrin and metalloproteinase domain-containing protein 17(Homo sapiens (Human))
Preclinical Research Novartis Pharma
Curated by ChEMBL
Preclinical Research Novartis Pharma
Curated by ChEMBL
Affinity DataKi: 0.600nMAssay Description:Inhibitory potency against Tumor necrosis factor alpha converting enzyme (TACE)More data for this Ligand-Target Pair
Target72 kDa type IV collagenase(Homo sapiens (Human))
Preclinical Research Novartis Pharma
Curated by ChEMBL
Preclinical Research Novartis Pharma
Curated by ChEMBL
Affinity DataKi: 0.600nMAssay Description:Inhibitory potency against Matrix metalloprotease-2 (MMP-2)More data for this Ligand-Target Pair
TargetInterstitial collagenase(Homo sapiens (Human))
Preclinical Research Novartis Pharma
Curated by ChEMBL
Preclinical Research Novartis Pharma
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Inhibitory potency against Matrix metalloprotease-1 (MMP-1)More data for this Ligand-Target Pair
Affinity DataKi: 0.900nMAssay Description:Inhibitory potency against Matrix metalloprotease-3 (MMP-3)More data for this Ligand-Target Pair
TargetInterstitial collagenase(Homo sapiens (Human))
Preclinical Research Novartis Pharma
Curated by ChEMBL
Preclinical Research Novartis Pharma
Curated by ChEMBL
Affinity DataKi: 1.10nMAssay Description:Inhibitory potency against Matrix metalloprotease-1 (MMP-1)More data for this Ligand-Target Pair
TargetInterstitial collagenase(Homo sapiens (Human))
Preclinical Research Novartis Pharma
Curated by ChEMBL
Preclinical Research Novartis Pharma
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Inhibitory potency against Matrix metalloprotease-1 (MMP-1)More data for this Ligand-Target Pair
TargetInterstitial collagenase(Homo sapiens (Human))
Preclinical Research Novartis Pharma
Curated by ChEMBL
Preclinical Research Novartis Pharma
Curated by ChEMBL
Affinity DataKi: 3.5nMAssay Description:Inhibitory potency against Matrix metalloprotease-1 (MMP-1)More data for this Ligand-Target Pair
TargetDisintegrin and metalloproteinase domain-containing protein 17(Homo sapiens (Human))
Preclinical Research Novartis Pharma
Curated by ChEMBL
Preclinical Research Novartis Pharma
Curated by ChEMBL
Affinity DataKi: 3.80nMAssay Description:Inhibitory potency against Tumor necrosis factor alpha converting enzyme (TACE)More data for this Ligand-Target Pair
Target72 kDa type IV collagenase(Homo sapiens (Human))
Preclinical Research Novartis Pharma
Curated by ChEMBL
Preclinical Research Novartis Pharma
Curated by ChEMBL
Affinity DataKi: 4.80nMAssay Description:Inhibitory potency against Matrix metalloprotease-2 (MMP-2)More data for this Ligand-Target Pair
TargetDisintegrin and metalloproteinase domain-containing protein 17(Homo sapiens (Human))
Preclinical Research Novartis Pharma
Curated by ChEMBL
Preclinical Research Novartis Pharma
Curated by ChEMBL
Affinity DataKi: 4.90nMAssay Description:Inhibitory potency against Tumor necrosis factor alpha converting enzyme (TACE)More data for this Ligand-Target Pair
TargetDisintegrin and metalloproteinase domain-containing protein 17(Homo sapiens (Human))
Preclinical Research Novartis Pharma
Curated by ChEMBL
Preclinical Research Novartis Pharma
Curated by ChEMBL
Affinity DataKi: 6.30nMAssay Description:Inhibitory potency against Tumor necrosis factor alpha converting enzyme (TACE)More data for this Ligand-Target Pair
Affinity DataKi: 8.90nMAssay Description:Inhibitory potency against Matrix metalloprotease-3 (MMP-3)More data for this Ligand-Target Pair
Affinity DataKi: 9.80nMAssay Description:Inhibitory potency against Matrix metalloprotease-3 (MMP-3)More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Inhibitory potency against Matrix metalloprotease-3 (MMP-3)More data for this Ligand-Target Pair
TargetDisintegrin and metalloproteinase domain-containing protein 17(Homo sapiens (Human))
Preclinical Research Novartis Pharma
Curated by ChEMBL
Preclinical Research Novartis Pharma
Curated by ChEMBL
Affinity DataKi: 13nMAssay Description:Inhibitory potency against Tumor necrosis factor alpha converting enzyme (TACE)More data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Inhibitory potency against Matrix metalloprotease-3 (MMP-3)More data for this Ligand-Target Pair
Affinity DataKi: 1.97E+3nMAssay Description:Competitive inhibition of human KDM2A expressed in Escherichia coli using 2-oxoglutarate by enzyme kinetic assayMore data for this Ligand-Target Pair
Affinity DataKi: 8.50E+4nMAssay Description:Mixed type inhibition of human KDM2A expressed in Escherichia coli assessed inhibition constant for compound-enzyme-substrate complex using methyl ly...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 0.0140nMAssay Description:Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
TargetCytoplasmic tyrosine-protein kinase BMX(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of BMX (unknown origin)More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 9(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at...More data for this Ligand-Target Pair
TargetMAP kinase-activated protein kinase 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assayMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 9(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Binding affinity determined by reduction in binding of 125 I-glucagon to the human glucagon receptor expressed on CHO cells in absence of Mg+2More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
TargetMAP kinase-activated protein kinase 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: <3nMAssay Description:Inhibition of MK2 mediated anisomycin-stimulated hsp27 phosphorylation in human THP-1 cells by fluorometric analysisChecked by AuthorMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair





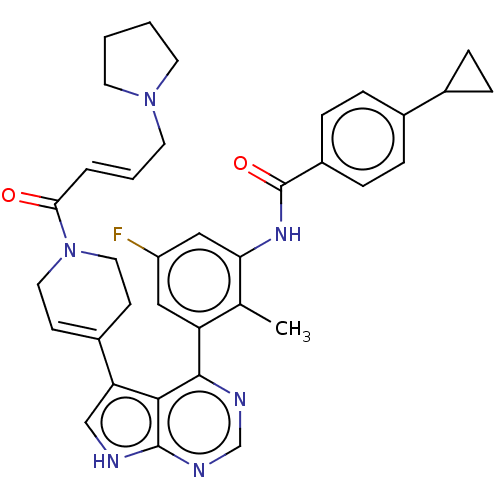

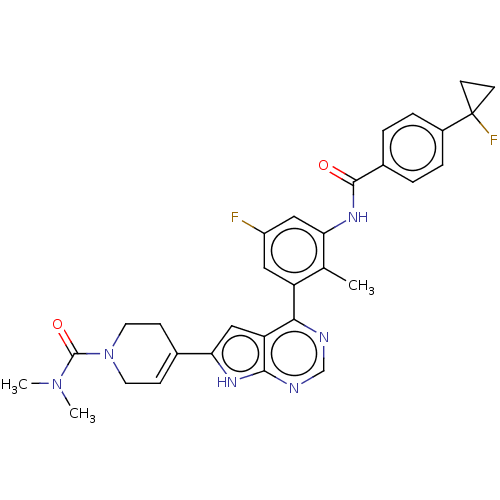
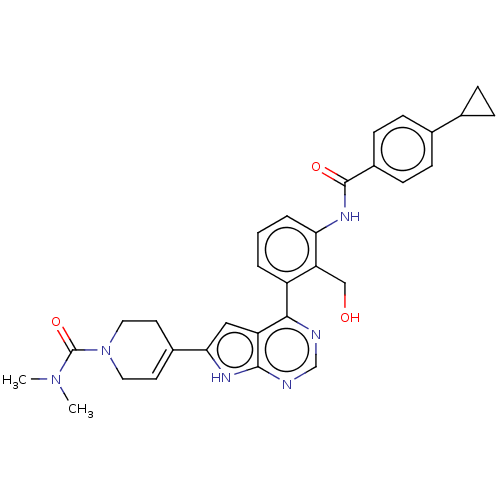
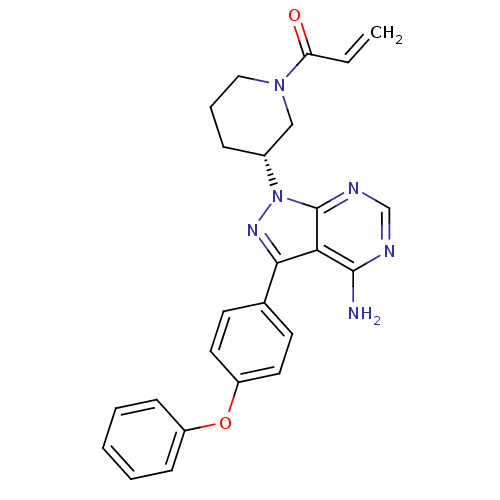






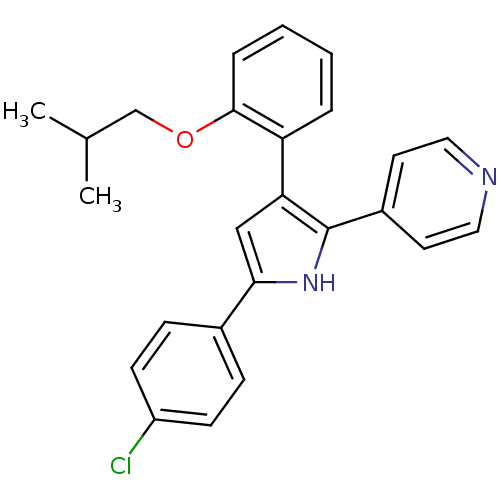
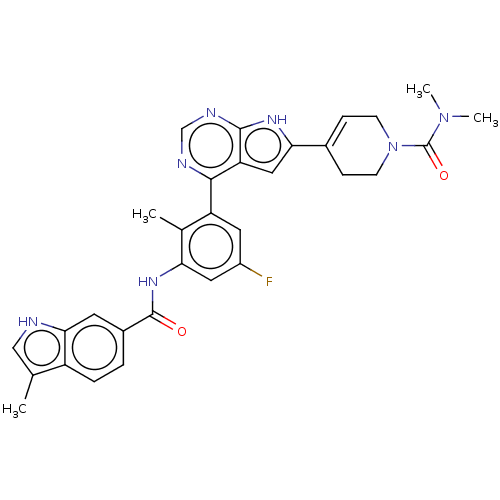
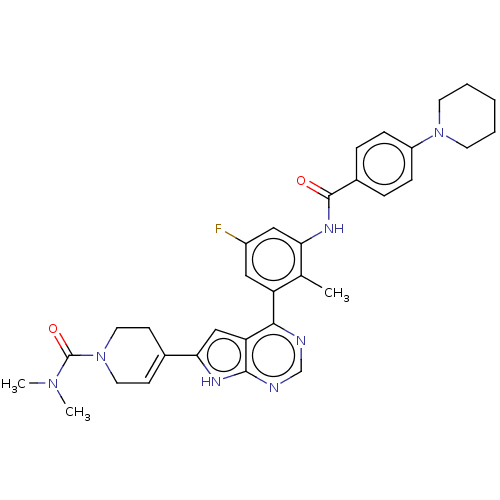
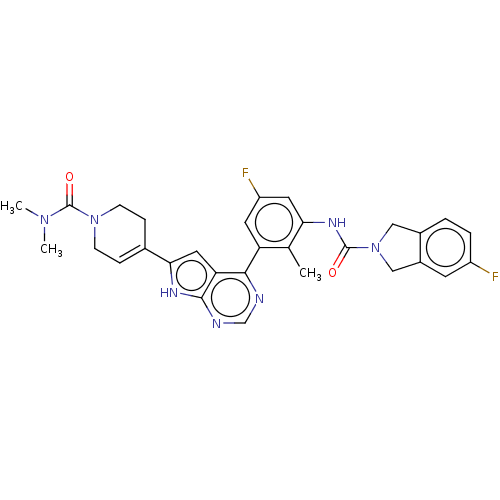
 3D Structure (crystal)
3D Structure (crystal)