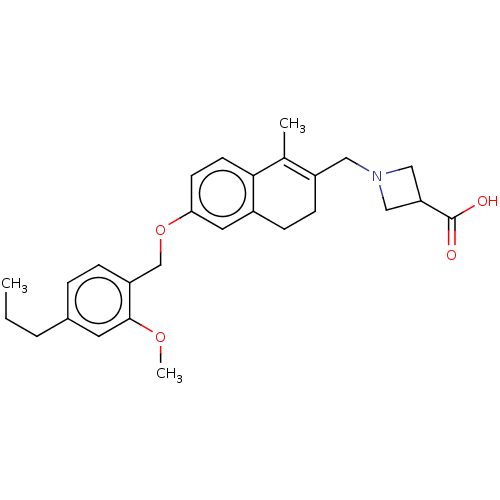
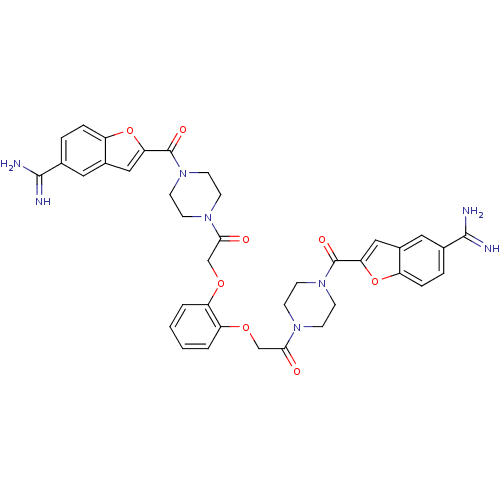
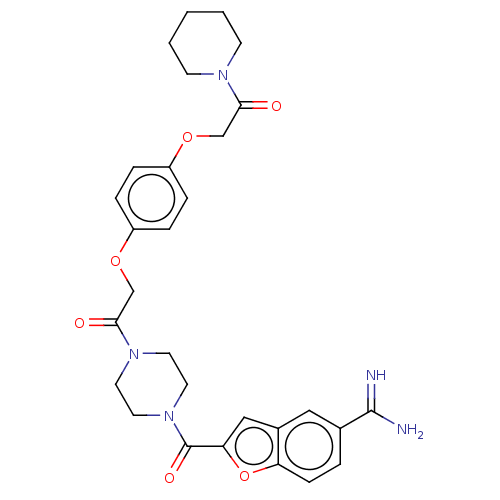
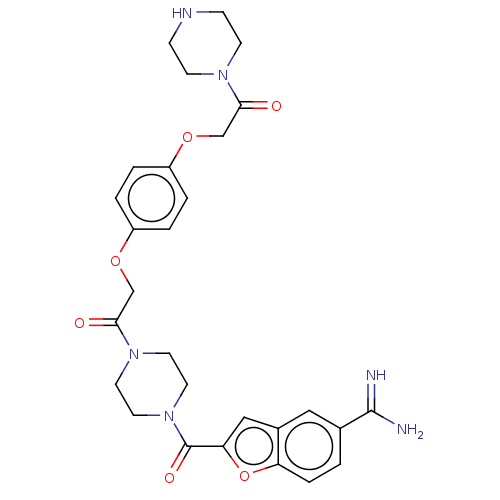
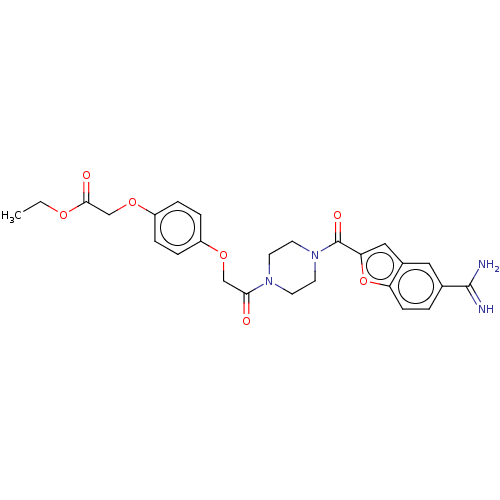
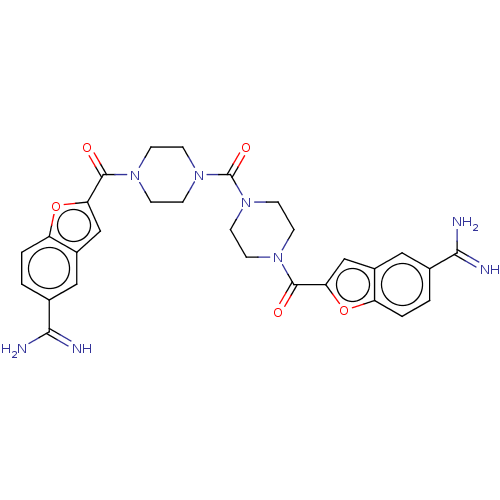
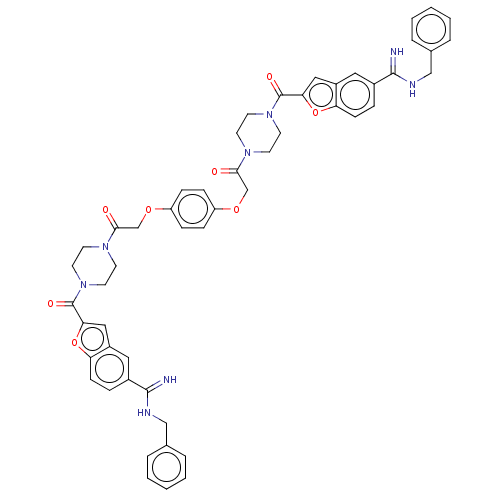
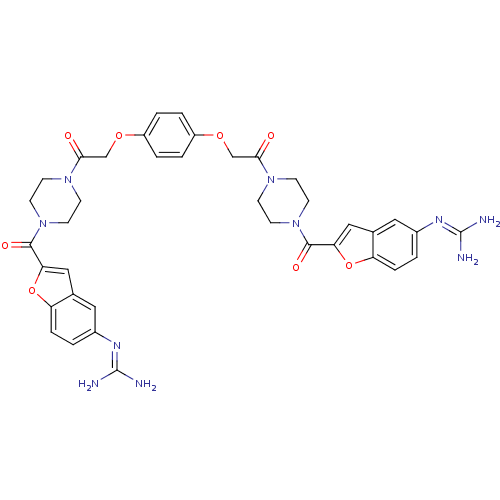
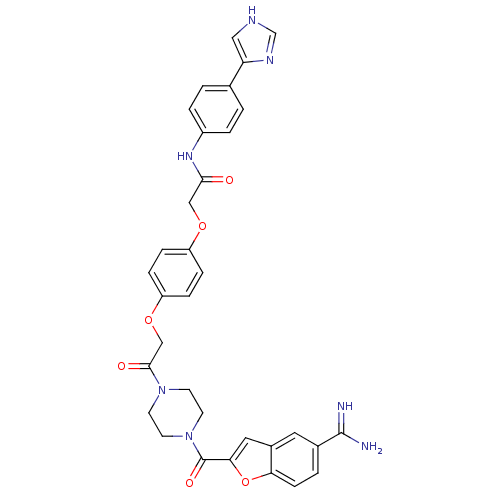
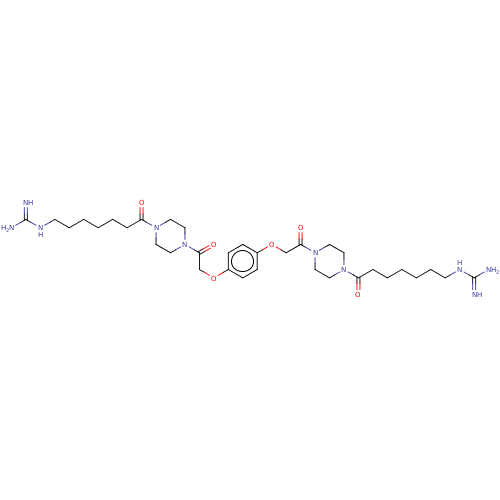
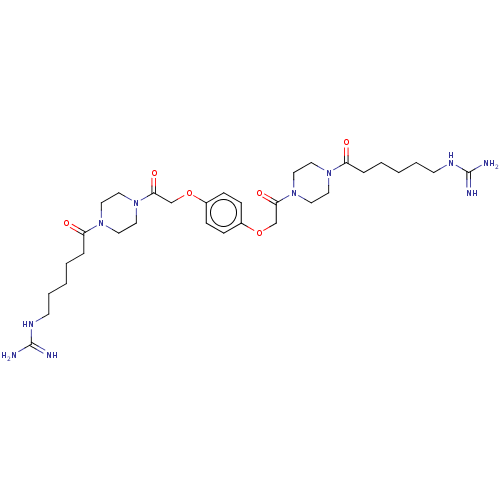
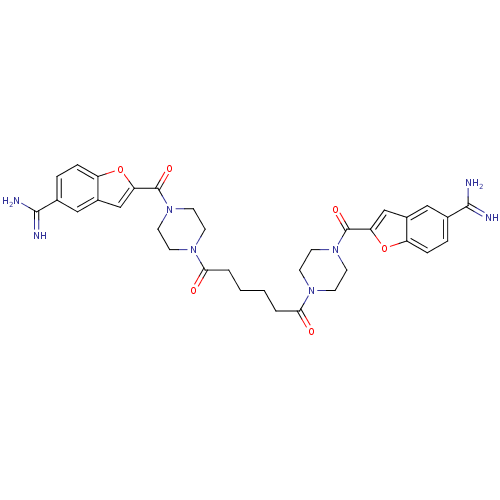
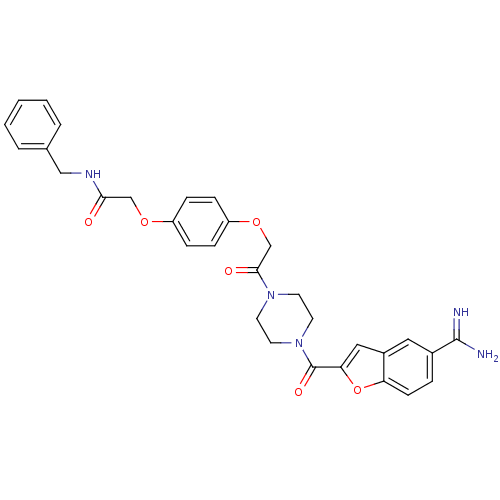
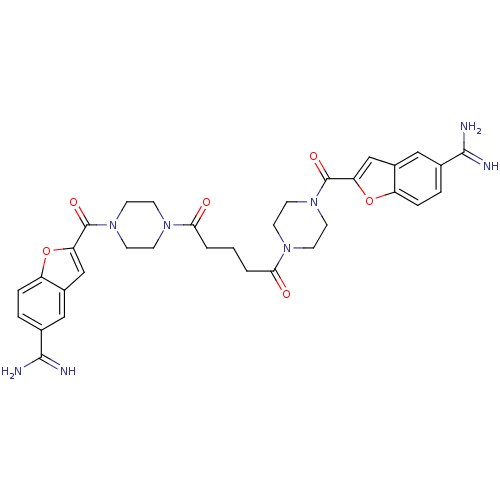
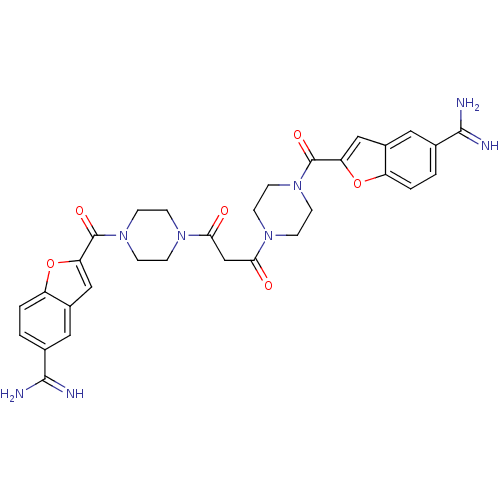
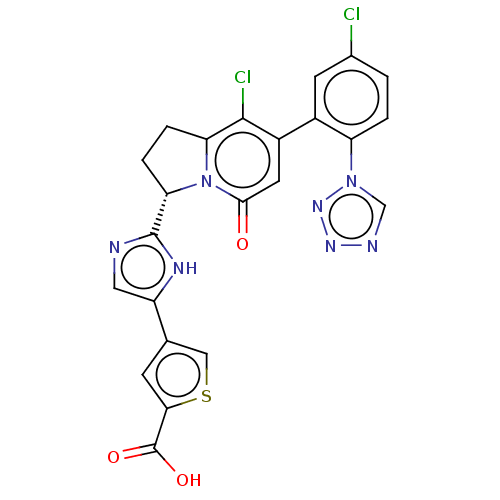
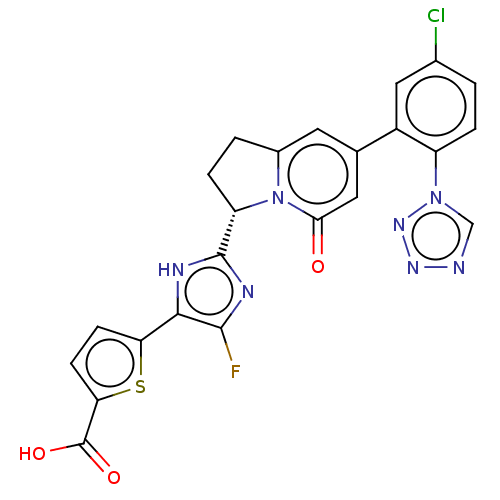
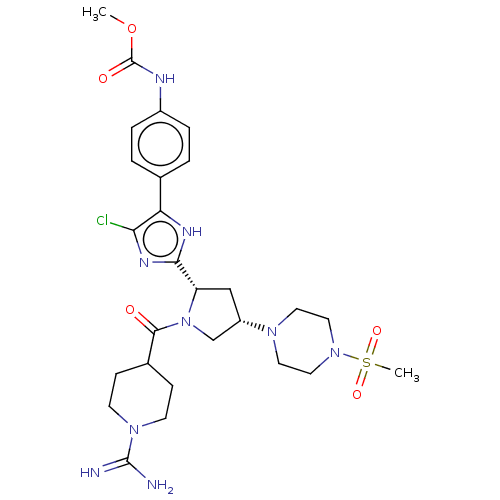
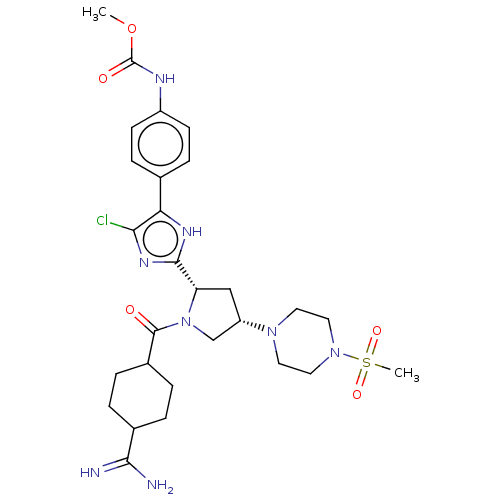
TargetTryptase beta-2/delta/gamma(Homo sapiens (Human))
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Affinity DataKi: 0.0190nMAssay Description:Inhibition of tryptase activityMore data for this Ligand-Target Pair
TargetTryptase beta-2/delta/gamma(Homo sapiens (Human))
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Affinity DataKi: 0.0280nMAssay Description:Inhibition of tryptase activityMore data for this Ligand-Target Pair
TargetTryptase beta-2/delta/gamma(Homo sapiens (Human))
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Affinity DataKi: 0.0290nMAssay Description:Inhibition of tryptase activityMore data for this Ligand-Target Pair
TargetTryptase beta-2/delta/gamma(Homo sapiens (Human))
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Affinity DataKi: 0.0460nMAssay Description:Inhibition of tryptase activityMore data for this Ligand-Target Pair
TargetTryptase beta-2/delta/gamma(Homo sapiens (Human))
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Affinity DataKi: 0.0570nMAssay Description:Inhibition of tryptase activityMore data for this Ligand-Target Pair
TargetTryptase beta-2/delta/gamma(Homo sapiens (Human))
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Affinity DataKi: 0.120nMAssay Description:Inhibition of tryptase activityMore data for this Ligand-Target Pair
Affinity DataKi: 0.574nMAssay Description:Displacement of [33P]-S1P from human S1P5 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 0.626nMAssay Description:Agonist activity at human S1P5 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP accumulation after 30 mins by E...More data for this Ligand-Target Pair
Affinity DataKi: 0.772nMAssay Description:Displacement of [33P]-S1P from human S1P2 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
TargetCorticotropin-releasing factor receptor 1(Homo sapiens (Human))
Minase Research Institute
Curated by ChEMBL
Minase Research Institute
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Displacement of [125I]CRF from human corticotropin-releasing factor receptor 1 expressed in CHO-K1 cells after 2 hrs by gamma countingMore data for this Ligand-Target Pair
TargetTryptase beta-2/delta/gamma(Homo sapiens (Human))
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Affinity DataKi: 1.10nMAssay Description:Inhibition of tryptase activityMore data for this Ligand-Target Pair
Affinity DataKi: 29nMAssay Description:Displacement of [33P]-S1P from human S1P4 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
TargetTryptase beta-2/delta/gamma(Homo sapiens (Human))
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Affinity DataKi: 60nMAssay Description:Inhibition of tryptase activityMore data for this Ligand-Target Pair
TargetTryptase beta-2/delta/gamma(Homo sapiens (Human))
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Affinity DataKi: 67nMAssay Description:Inhibition of tryptase activityMore data for this Ligand-Target Pair
TargetTryptase beta-2/delta/gamma(Homo sapiens (Human))
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Affinity DataKi: 210nMAssay Description:Inhibition of tryptase activityMore data for this Ligand-Target Pair
TargetTryptase beta-2/delta/gamma(Homo sapiens (Human))
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Affinity DataKi: 460nMAssay Description:Inhibition of tryptase activityMore data for this Ligand-Target Pair
TargetTryptase beta-2/delta/gamma(Homo sapiens (Human))
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Affinity DataKi: 970nMAssay Description:Inhibition of tryptase activityMore data for this Ligand-Target Pair
TargetTryptase beta-2/delta/gamma(Homo sapiens (Human))
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Affinity DataKi: 1.10E+3nMAssay Description:Inhibition of tryptase activityMore data for this Ligand-Target Pair
TargetTryptase beta-2/delta/gamma(Homo sapiens (Human))
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Affinity DataKi: 1.10E+3nMAssay Description:Inhibition of tryptase activityMore data for this Ligand-Target Pair
TargetTryptase beta-2/delta/gamma(Homo sapiens (Human))
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Affinity DataKi: 3.00E+3nMAssay Description:Inhibition of tryptase activityMore data for this Ligand-Target Pair
Affinity DataKi: 5.30E+3nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataKi: >5.45E+3nMAssay Description:Displacement of [33P]-S1P from rat S1P1 receptor after 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: >5.63E+3nMAssay Description:Displacement of [33P]-S1P from human S1P3 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
TargetTryptase beta-2/delta/gamma(Homo sapiens (Human))
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Affinity DataKi: 6.60E+3nMAssay Description:Inhibition of tryptase activityMore data for this Ligand-Target Pair
TargetTryptase beta-2/delta/gamma(Homo sapiens (Human))
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Affinity DataKi: 1.30E+4nMAssay Description:Inhibition of tryptase activityMore data for this Ligand-Target Pair
TargetTryptase beta-2/delta/gamma(Homo sapiens (Human))
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Affinity DataKi: 1.30E+4nMAssay Description:Inhibition of tryptase activityMore data for this Ligand-Target Pair
Affinity DataKi: 1.50E+4nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 2.20E+4nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
TargetTryptase beta-2/delta/gamma(Homo sapiens (Human))
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Affinity DataKi: 2.30E+4nMAssay Description:Inhibition of tryptase activityMore data for this Ligand-Target Pair
Affinity DataKi: 2.30E+4nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 2.40E+4nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 2.80E+4nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 2.90E+4nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 3.20E+4nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 3.20E+4nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 5.10E+4nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
TargetTryptase beta-2/delta/gamma(Homo sapiens (Human))
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Yoshitomi Pharmaceutical Industries
Curated by ChEMBL
Affinity DataKi: 8.00E+4nMAssay Description:Inhibition of tryptase activityMore data for this Ligand-Target Pair
Affinity DataKi: 8.20E+4nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 8.50E+4nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 8.80E+4nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 2.30E+5nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Human Factor XIa (Haematologic Technologies Inc.) activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Human Factor XIa (Haematologic Technologies Inc.) activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Human Factor XIa activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6000, 50 mM HEPES-NaOH (pH7.4) wit...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Human Factor XIa activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6000, 50 mM HEPES-NaOH (pH7.4) wit...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Human Factor XIa (Haematologic Technologies Inc.) activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Inhibitory activities of compounds of the present invention against factor XIa, Xa, XIIa, IXa, VIIa, plasma kallikrein or thrombin were evaluated usi...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Human Factor XIa (Haematologic Technologies Inc.) activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Human Factor XIa (Haematologic Technologies Inc.) activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Human Factor XIa activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6000, 50 mM HEPES-NaOH (pH7.4) wit...More data for this Ligand-Target Pair