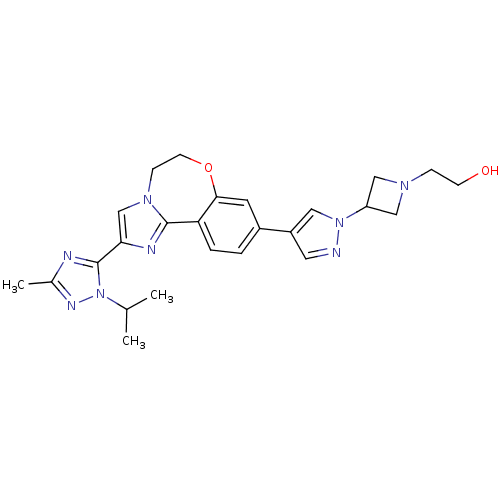
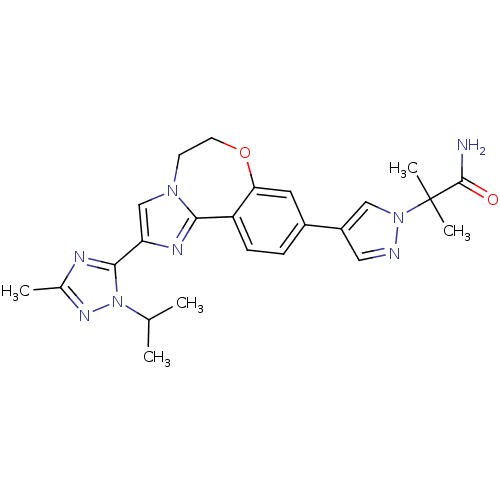
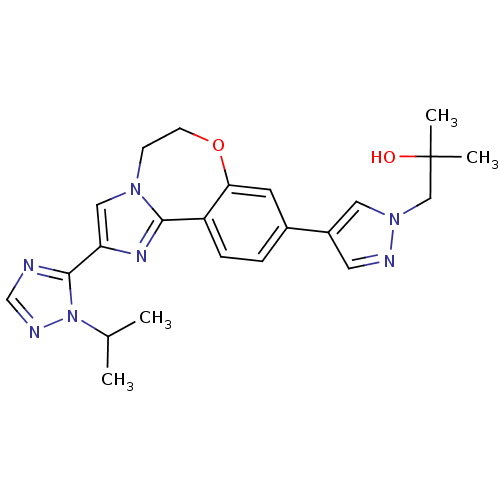
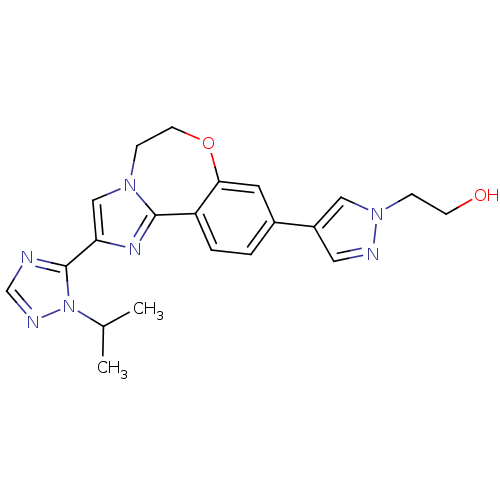
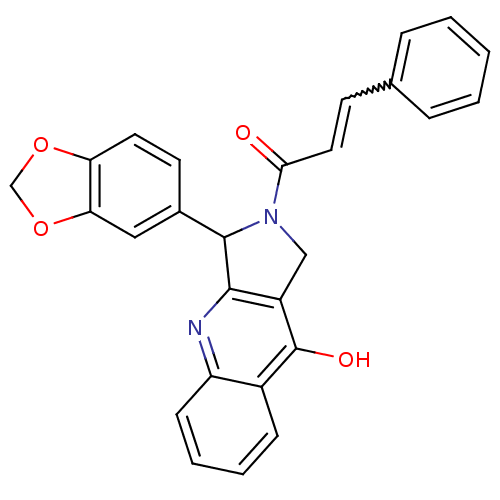
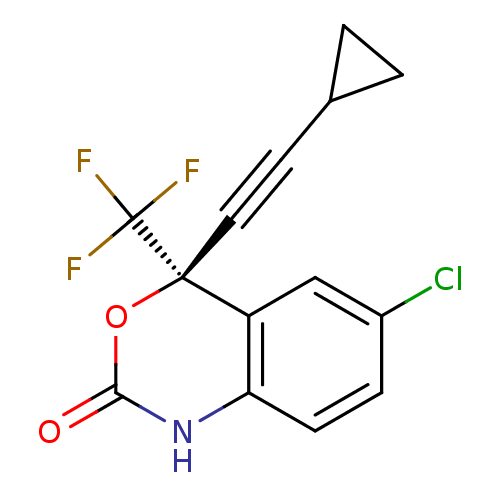
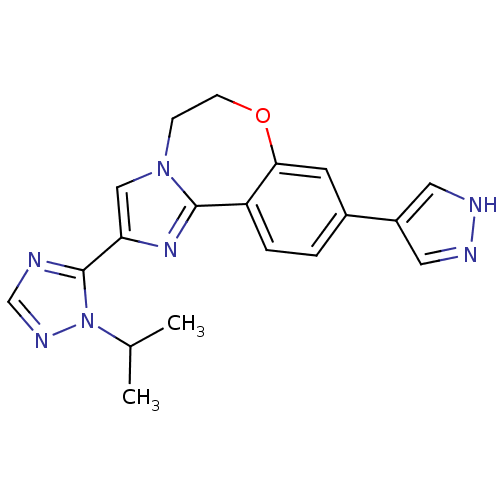
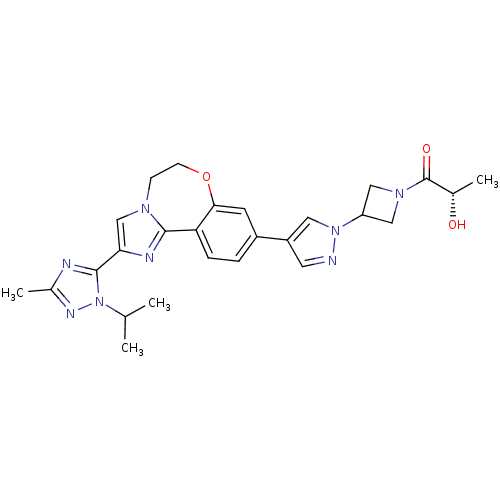
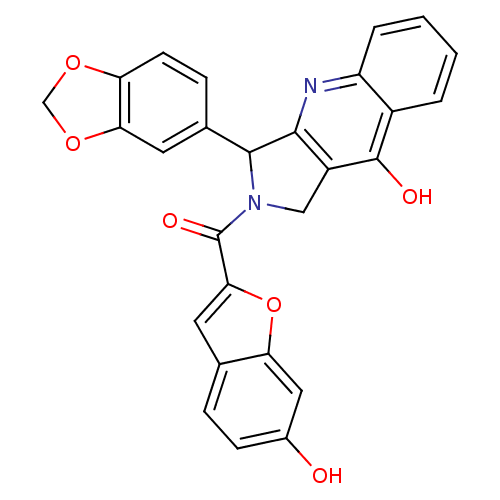
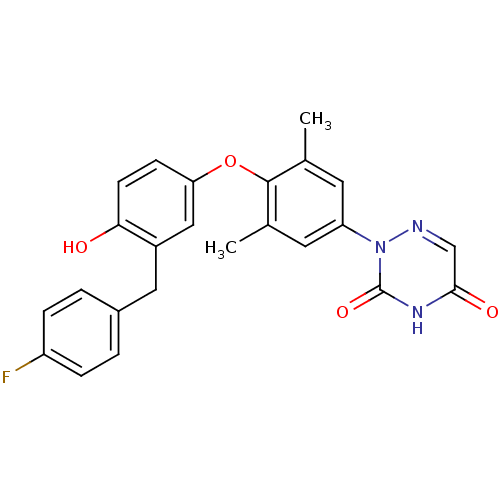
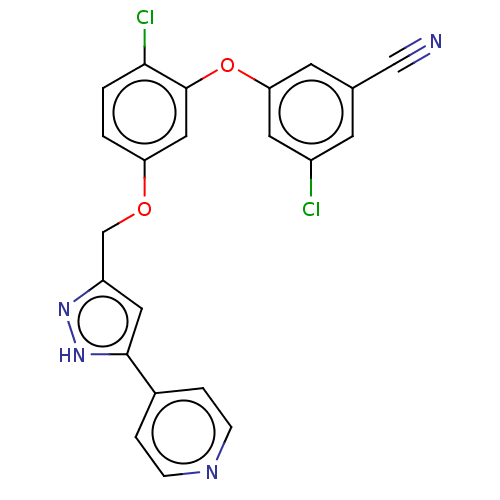
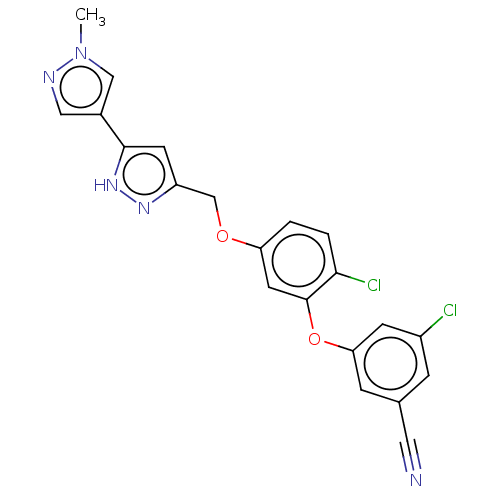
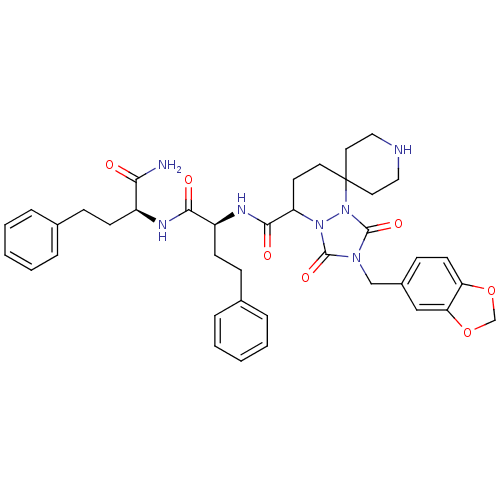
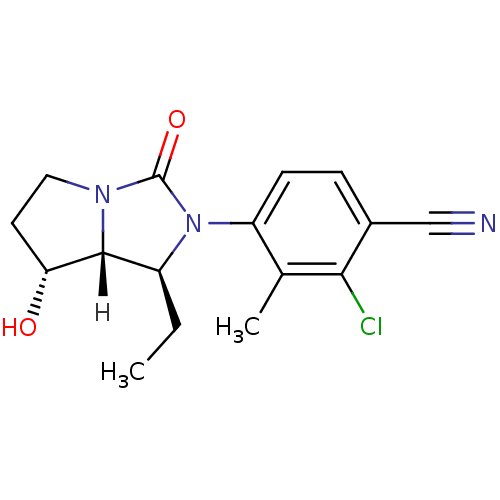
Affinity DataKi: 0.0510nM ΔG°: -58.7kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NAD+More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.110nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.120nMAssay Description:Inhibition of PI3Kdelta (unknown origin)More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.140nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.150nMAssay Description:Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluatedMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.170nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.200nMAssay Description:Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluatedMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.220nMAssay Description:Inhibitory activity against human Phosphodiesterase 5More data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.230nMAssay Description:Inhibitory activity against human Phosphodiesterase 5More data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.240nMAssay Description:Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluatedMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.290nMAssay Description:Inhibitory activity against human Phosphodiesterase 5More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.290nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.290nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Merck Research Laboratory
Curated by ChEMBL
Merck Research Laboratory
Curated by ChEMBL
Affinity DataKi: 0.300nMAssay Description:Inhibition of wild type HIV1 reverse transcriptase by SPA assayMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Merck Research Laboratory
Curated by ChEMBL
Merck Research Laboratory
Curated by ChEMBL
Affinity DataKi: 0.300nMAssay Description:Inhibition of wild type HIV1 reverse transcriptase by SPA assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.300nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Merck Research Laboratory
Curated by ChEMBL
Merck Research Laboratory
Curated by ChEMBL
Affinity DataKi: 0.300nMAssay Description:Inhibition of HIV1 reverse transcriptase Y181C mutant by SPA assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.310nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.310nMAssay Description:Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluatedMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.340nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.350nMAssay Description:Inhibitory activity against human Phosphodiesterase 5More data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.350nMAssay Description:Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluatedMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Merck Research Laboratory
Curated by ChEMBL
Merck Research Laboratory
Curated by ChEMBL
Affinity DataKi: 0.400nMAssay Description:Inhibition of wild type HIV1 reverse transcriptase by SPA assayMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Merck Research Laboratory
Curated by ChEMBL
Merck Research Laboratory
Curated by ChEMBL
Affinity DataKi: 0.400nMAssay Description:Inhibition of wild type HIV1 reverse transcriptase by SPA assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Binding affinity to thyroid receptor betaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.400nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
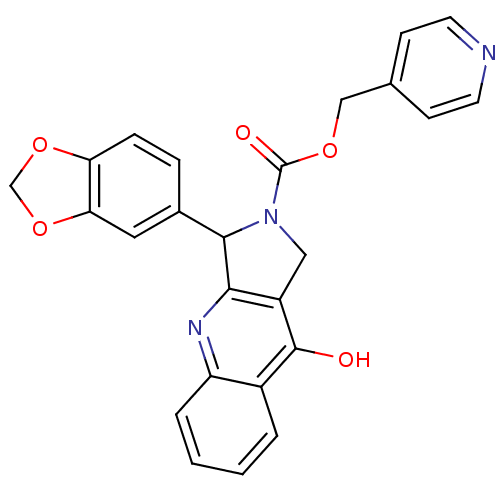
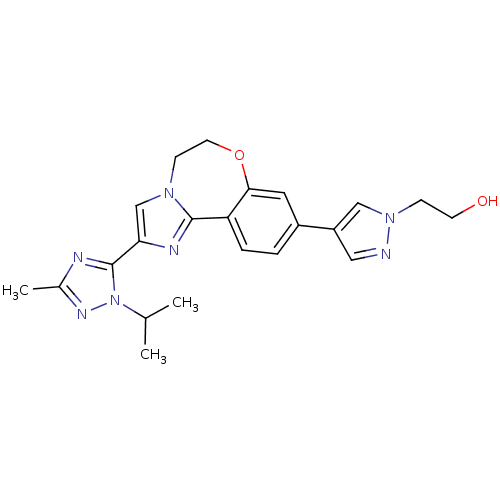
Affinity DataKi: 0.440nM ΔG°: -53.4kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NAD+More data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.470nMAssay Description:Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluatedMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Merck Research Laboratory
Curated by ChEMBL
Merck Research Laboratory
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:Inhibition of wild type HIV1 reverse transcriptase by SPA assayMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Merck Research Laboratory
Curated by ChEMBL
Merck Research Laboratory
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:Inhibition of HIV1 reverse transcriptase Y181C mutant by electrochemiluminescent assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Binding affinity to thyroid receptor betaMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Binding affinity to thyroid receptor betaMore data for this Ligand-Target Pair
TargetReverse transcriptase protein(Human immunodeficiency virus 1)
Merck Research Laboratory
Curated by ChEMBL
Merck Research Laboratory
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nM ΔG°: -52.6kJ/mole EC50: 2nMpH: 7.4 T: 2°CAssay Description:Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E...More data for this Ligand-Target Pair
Affinity DataKi: 0.510nMAssay Description:Binding affinity to thyroid receptor betaMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.530nMAssay Description:Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluatedMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.580nMAssay Description:Inhibitory activity against human Phosphodiesterase 5More data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Merck Research Laboratory
Curated by ChEMBL
Merck Research Laboratory
Curated by ChEMBL
Affinity DataKi: 0.600nMAssay Description:Inhibition of HIV1 reverse transcriptase Y181C mutant by electrochemiluminescent assayMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Merck Research Laboratory
Curated by ChEMBL
Merck Research Laboratory
Curated by ChEMBL
Affinity DataKi: 0.600nMAssay Description:Inhibition of HIV1 reverse transcriptase Y181C mutant by electrochemiluminescent assayMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.610nMAssay Description:Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluatedMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.610nMAssay Description:Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluatedMore data for this Ligand-Target Pair
TargetReverse transcriptase protein(Human immunodeficiency virus 1)
Merck Research Laboratory
Curated by ChEMBL
Merck Research Laboratory
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assayMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Merck Research Laboratory
Curated by ChEMBL
Merck Research Laboratory
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Inhibition of wild type HIV1 reverse transcriptase by SPA assayMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Merck Research Laboratory
Curated by ChEMBL
Merck Research Laboratory
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Inhibition of wild type HIV1 reverse transcriptase by SPA assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:Binding affinity to thyroid receptor alphaMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Merck Research Laboratory
Curated by ChEMBL
Merck Research Laboratory
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Inhibition of wild type HIV1 reverse transcriptase by SPA assayMore data for this Ligand-Target Pair
TargetMotilin receptor(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:In vitro binding affinity towards human motilin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.700nM ΔG°: -51.7kJ/mole EC50: 2.60nMpH: 7.4 T: 2°CAssay Description:Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E...More data for this Ligand-Target Pair
TargetReverse transcriptase protein(Human immunodeficiency virus 1)
Merck Research Laboratory
Curated by ChEMBL
Merck Research Laboratory
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assayMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.710nMAssay Description:Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluatedMore data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)