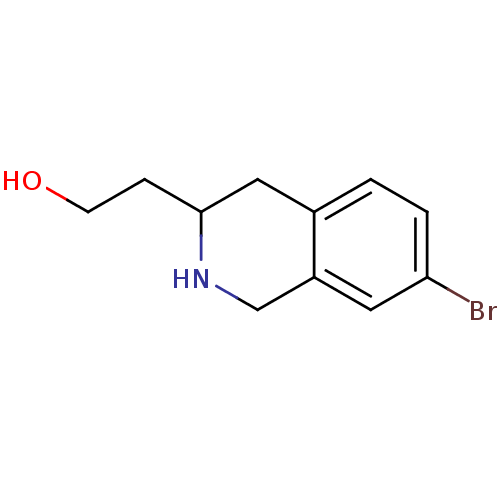
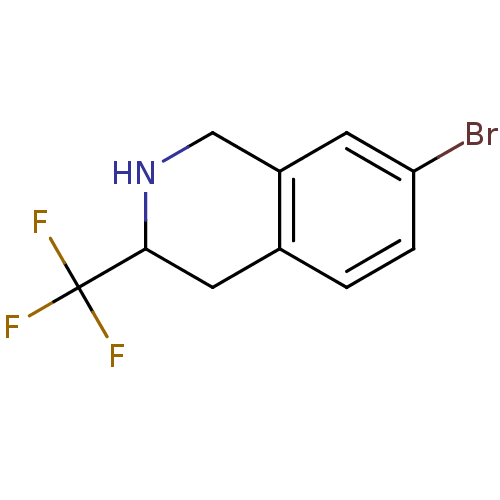
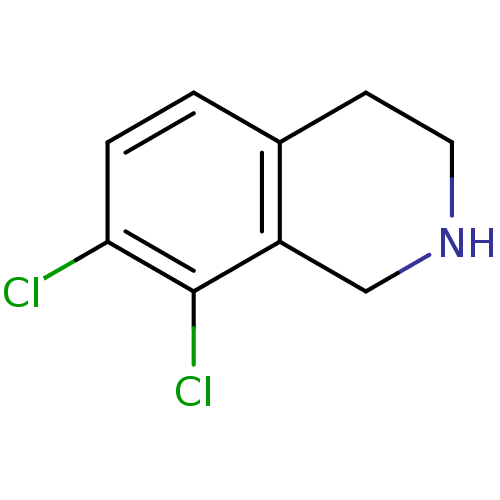
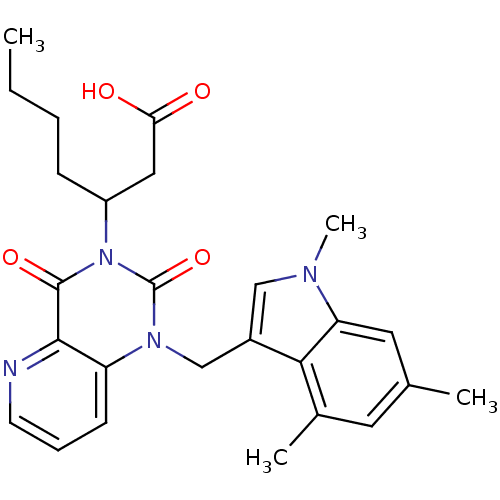
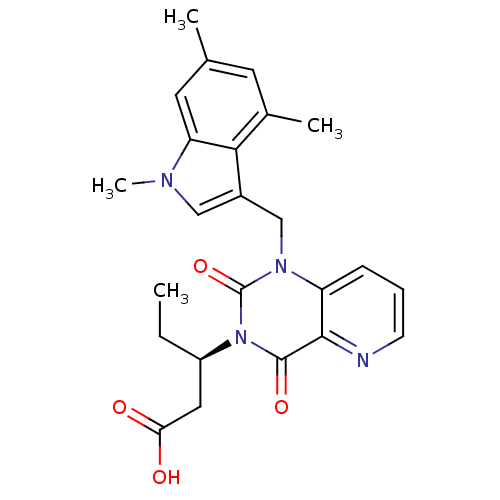
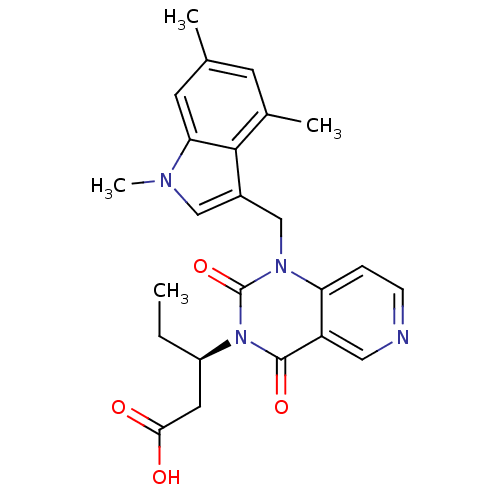
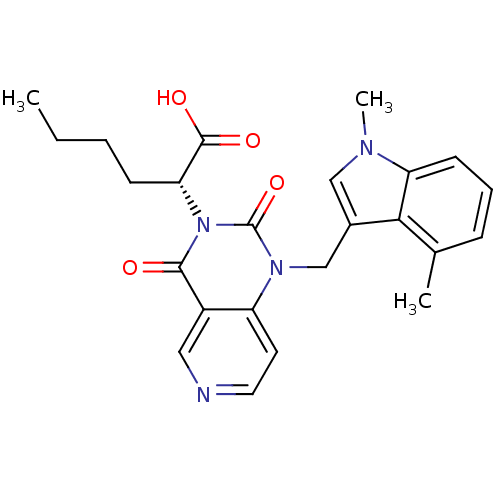
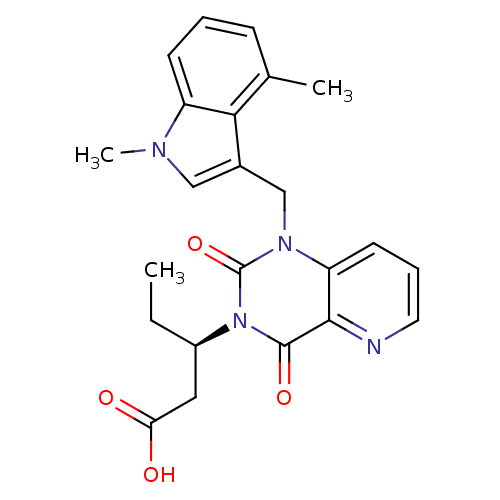
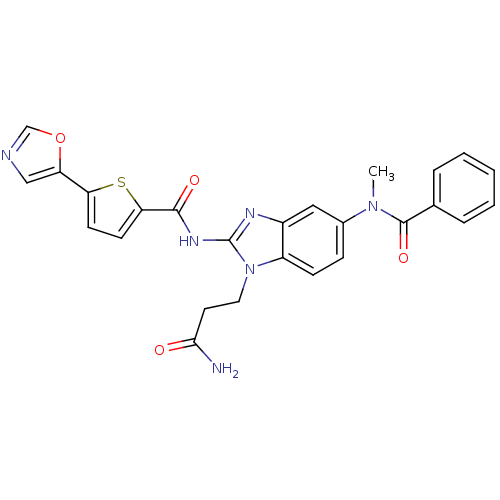
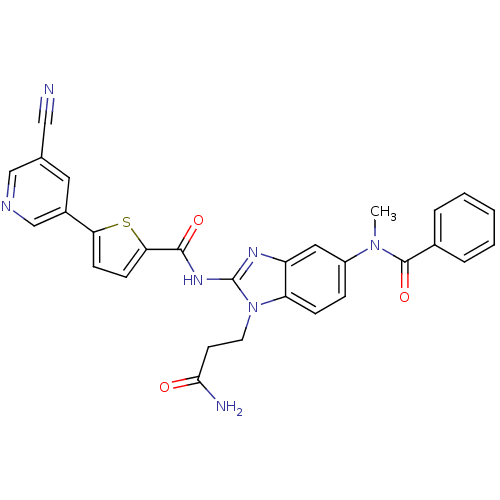
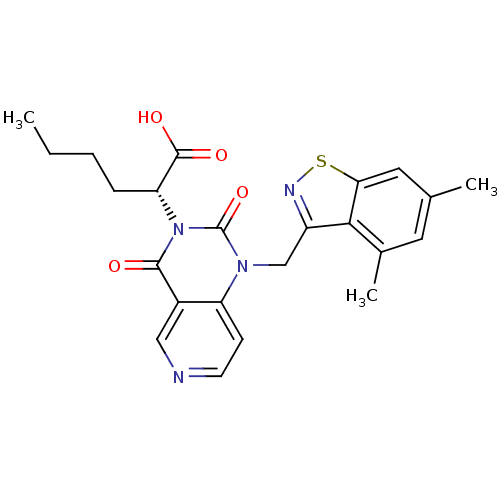
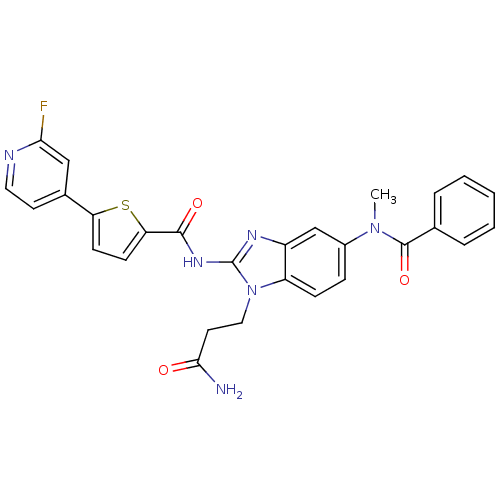
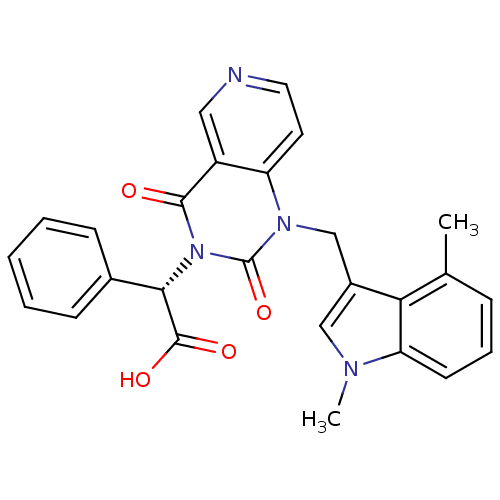
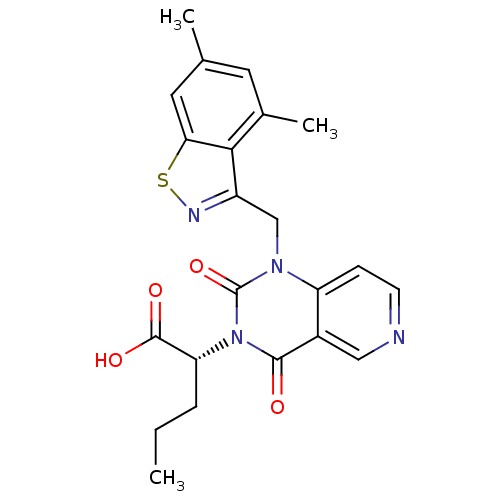
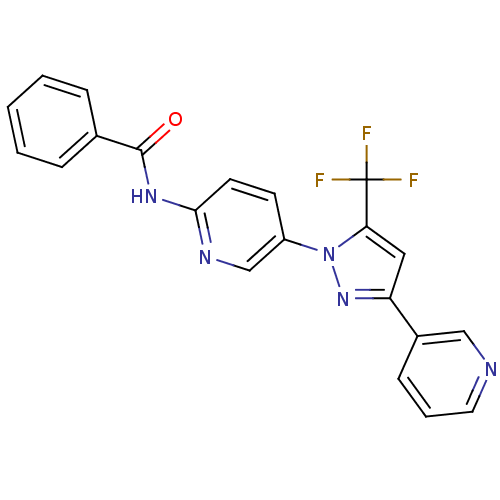
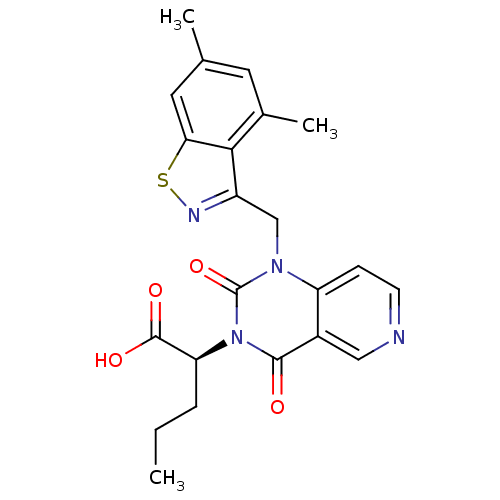
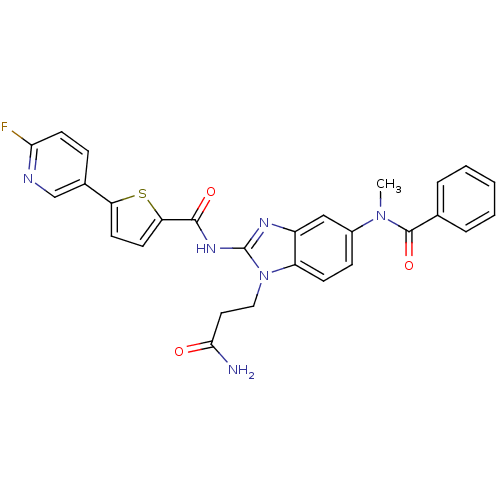
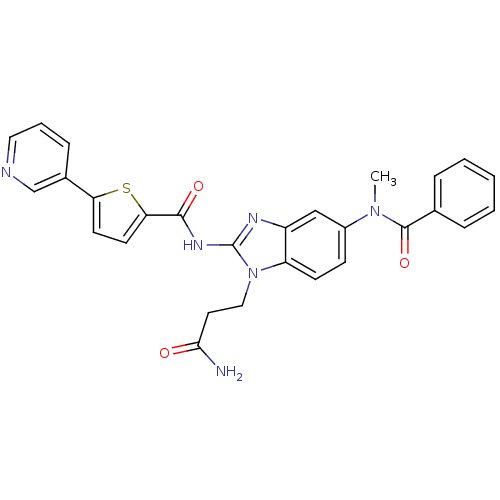
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
University Of Kansas
Curated by ChEMBL
University Of Kansas
Curated by ChEMBL
Affinity DataKi: 12nMAssay Description:Binding affinity against Human phenylethanolamine N-methyltransferaseMore data for this Ligand-Target Pair
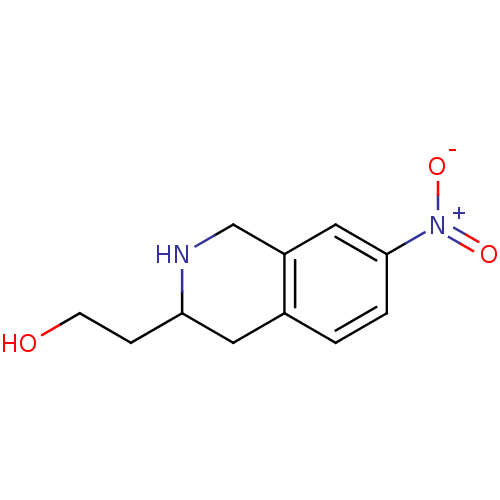
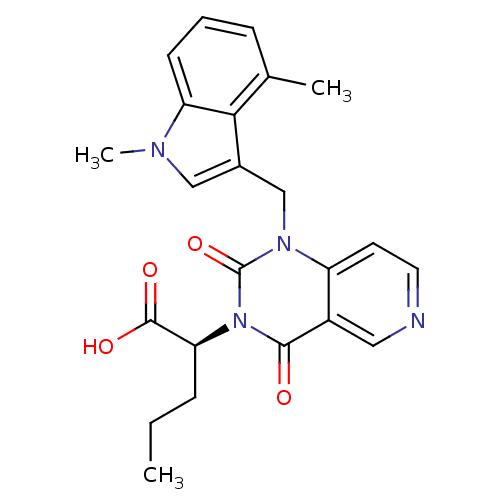
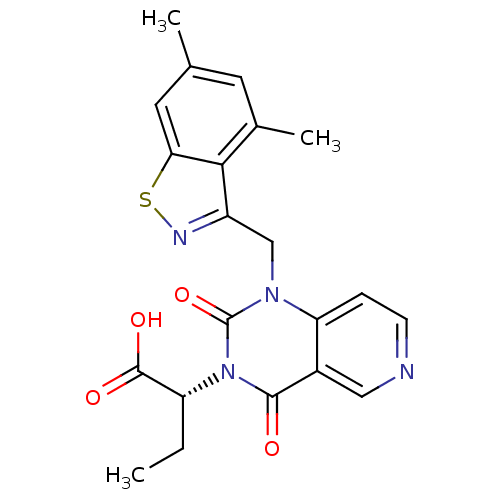
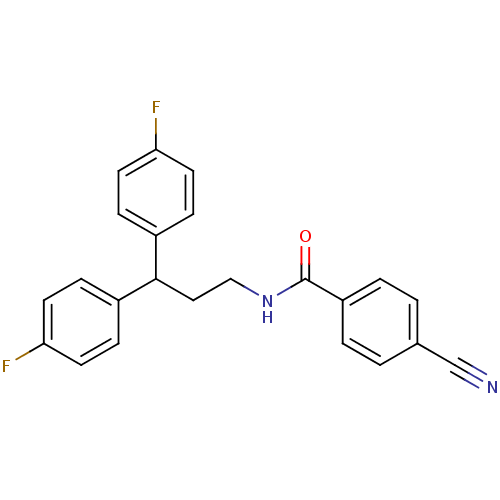
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
University Of Kansas
Curated by ChEMBL
University Of Kansas
Curated by ChEMBL
Affinity DataKi: 47nMAssay Description:Binding affinity against Human phenylethanolamine N-methyltransferaseMore data for this Ligand-Target Pair
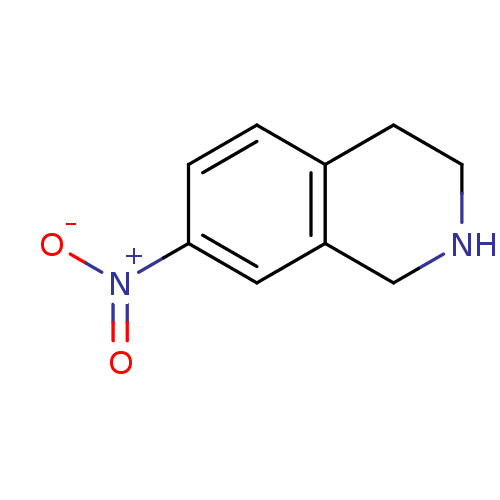
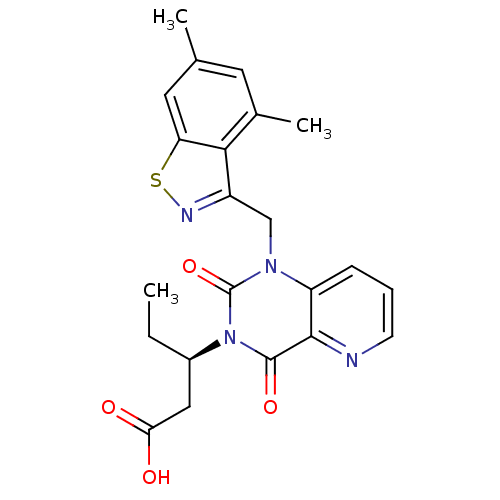
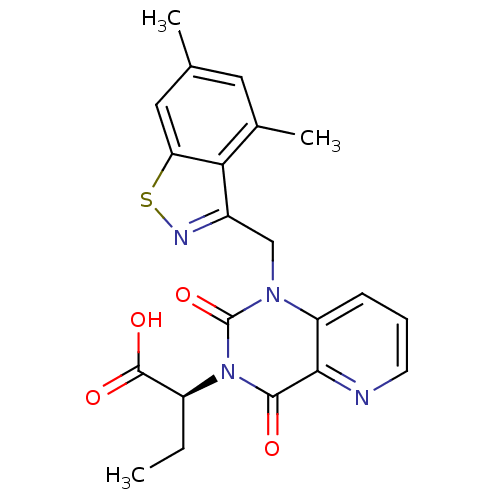
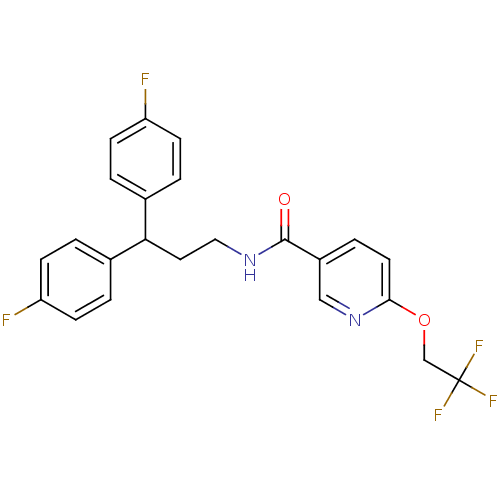
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
University Of Kansas
Curated by ChEMBL
University Of Kansas
Curated by ChEMBL
Affinity DataKi: 350nMAssay Description:Binding affinity against Human phenylethanolamine N-methyltransferaseMore data for this Ligand-Target Pair
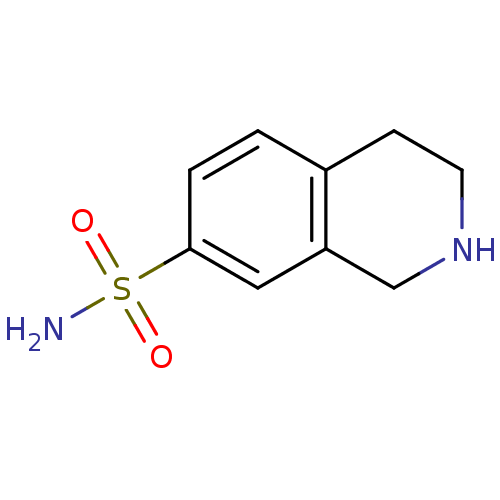
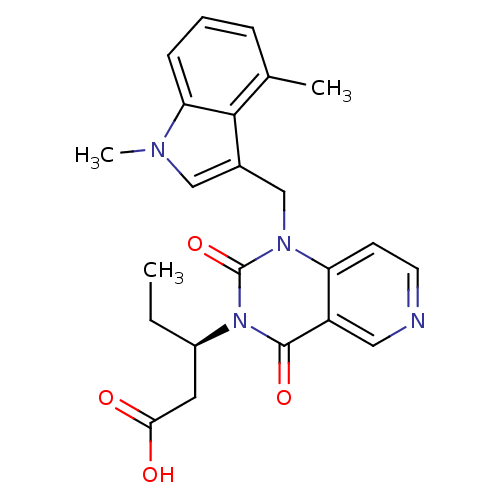
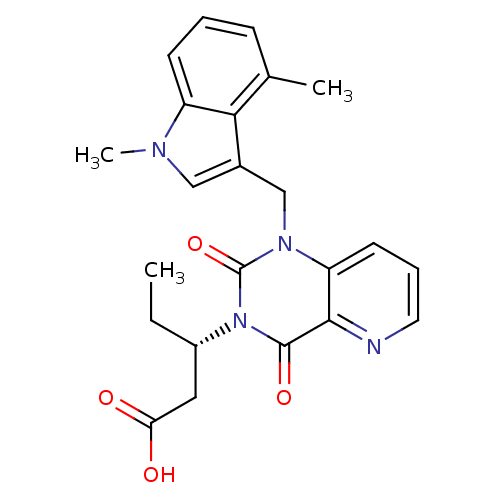
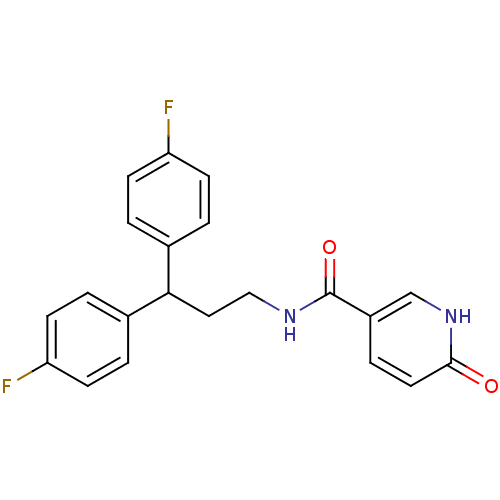
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
University Of Kansas
Curated by ChEMBL
University Of Kansas
Curated by ChEMBL
Affinity DataKi: 510nMAssay Description:Binding affinity against Human phenylethanolamine N-methyltransferaseMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
University Of Kansas
Curated by ChEMBL
University Of Kansas
Curated by ChEMBL
Affinity DataKi: 1.40E+3nMAssay Description:Binding affinity against Human phenylethanolamine N-methyltransferaseMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
University Of Kansas
Curated by ChEMBL
University Of Kansas
Curated by ChEMBL
Affinity DataKi: 1.70E+3nMAssay Description:Binding affinity against Human phenylethanolamine N-methyltransferaseMore data for this Ligand-Target Pair
Affinity DataKi: 3.20E+3nMAssay Description:Binding affinity against human alpha 2A adrenergic receptor in CHO cells using [3H]-RX-821002 as radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 3.20E+3nMAssay Description:Binding affinity against human alpha 2A adrenergic receptor in CHO cells using [3H]-RX-821002 as radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 3.40E+3nMAssay Description:Binding affinity against human alpha 2A adrenergic receptor in CHO cells using [3H]-RX-821002 as radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 3.40E+3nMAssay Description:Binding affinity against human alpha 2A adrenergic receptor in CHO cells using [3H]-RX-821002 as radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 3.60E+3nMAssay Description:Binding affinity against human alpha 2A adrenergic receptor in CHO cells using [3H]-RX-821002 as radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 3.90E+3nMAssay Description:Binding affinity against human alpha 2A adrenergic receptor in CHO cells using [3H]-RX-821002 as radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 4.35E+3nMAssay Description:Binding affinity against human alpha 2A adrenergic receptor in CHO cells using [3H]-RX-821002 as radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 4.84E+3nMAssay Description:Binding affinity against human alpha 2A adrenergic receptor in CHO cells using [3H]-RX-821002 as radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 6.52E+3nMAssay Description:Binding affinity against human alpha 2A adrenergic receptor in CHO cells using [3H]-RX-821002 as radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Displacement of [3H]-cDPPO from human Ephx2 by cell-based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 2nMpH: 7.0 T: 2°CAssay Description:Kinase is purified as a GST-fusion protein. The kinase activity is measured using DELFIA which utilizes europium chelate-labeled anti-phosphotyrosine...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMpH: 7.0 T: 2°CAssay Description:Kinase is purified as a GST-fusion protein. The kinase activity is measured using DELFIA which utilizes europium chelate-labeled anti-phosphotyrosine...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Kinase is purified as a GST-fusion protein. The kinase activity is measured using DELFIA which utilizes europium chelate-labeled anti-phosphotyrosine...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Kinase is purified as a GST-fusion protein. The kinase activity is measured using DELFIA which utilizes europium chelate-labeled anti-phosphotyrosine...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Kinase is purified as a GST-fusion protein. The kinase activity is measured using DELFIA which utilizes europium chelate-labeled anti-phosphotyrosine...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 2.70nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 3nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Kinase is purified as a GST-fusion protein. The kinase activity is measured using DELFIA which utilizes europium chelate-labeled anti-phosphotyrosine...More data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 4nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 4.70nMAssay Description:Inhibition of human Ephx2 after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.80nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Rattus norvegicus)
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 4.80nMAssay Description:Displacement of rhodamine-labeled probe from rat soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Kinase is purified as a GST-fusion protein. The kinase activity is measured using DELFIA which utilizes europium chelate-labeled anti-phosphotyrosine...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMpH: 7.0 T: 2°CAssay Description:Kinase is purified as a GST-fusion protein. The kinase activity is measured using DELFIA which utilizes europium chelate-labeled anti-phosphotyrosine...More data for this Ligand-Target Pair

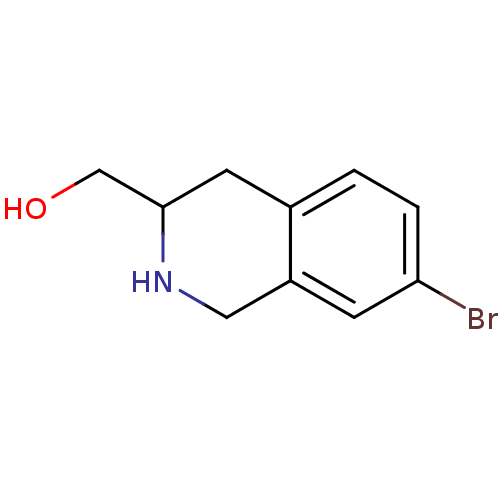
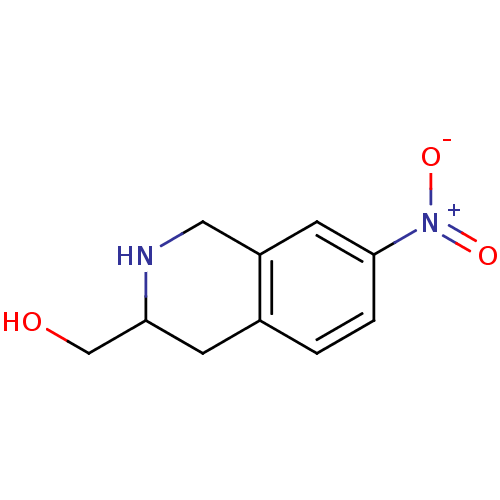
 3D Structure (crystal)
3D Structure (crystal)