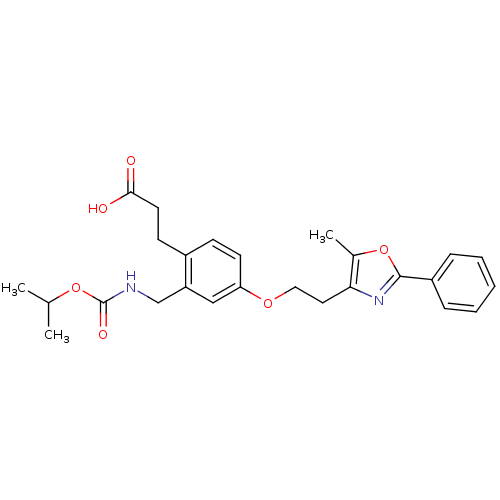
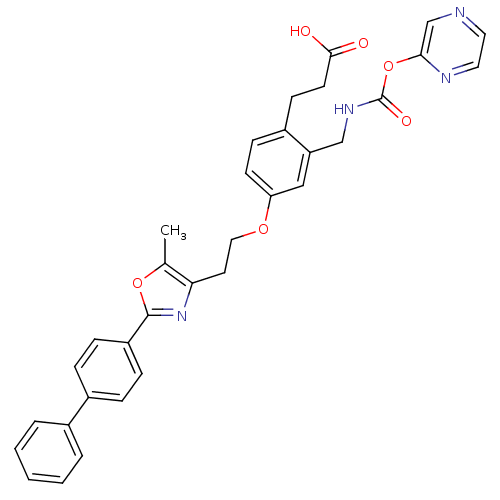
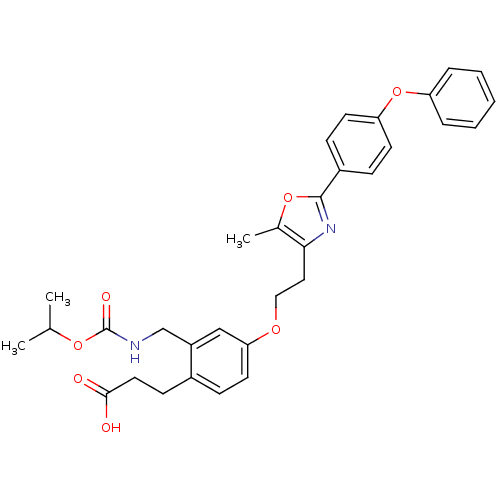
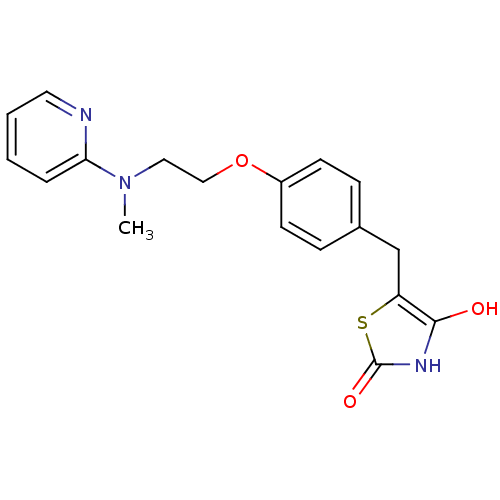
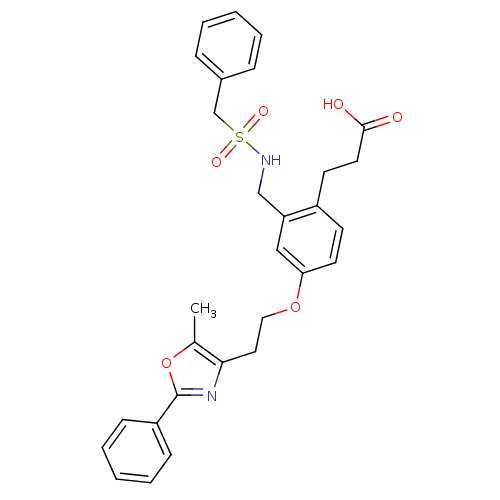
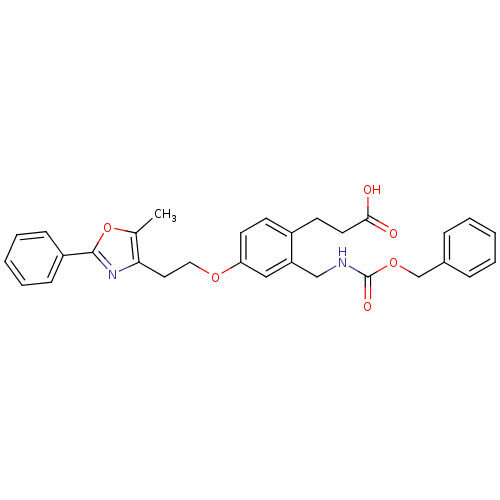
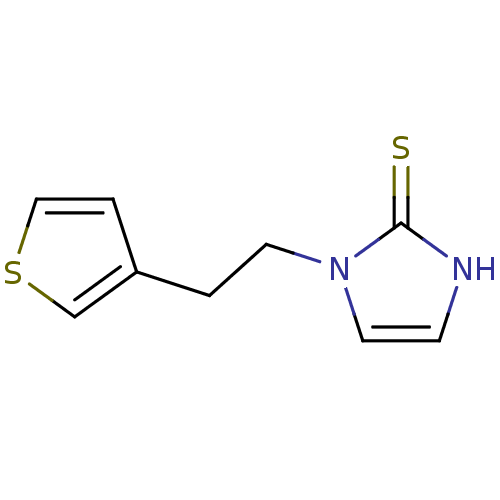
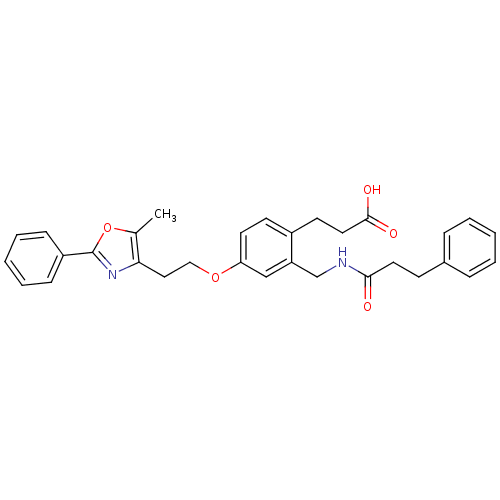
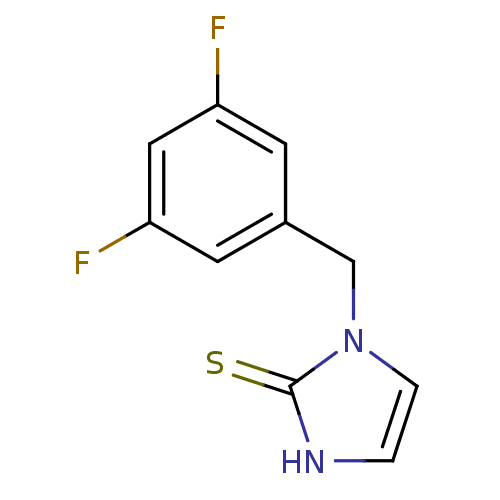
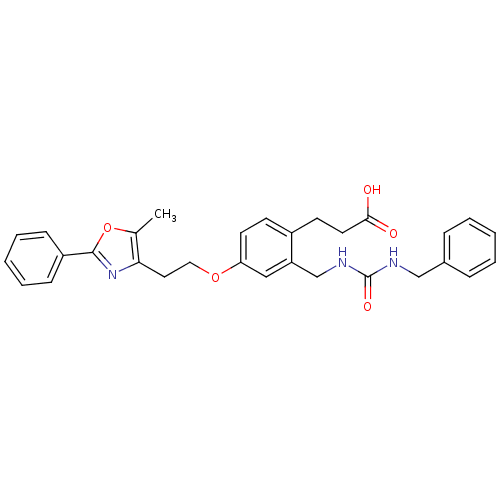
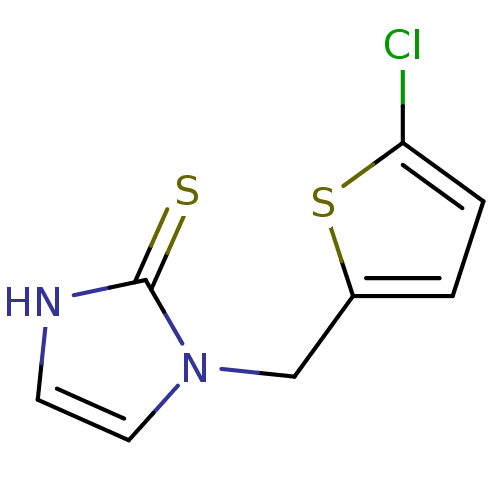
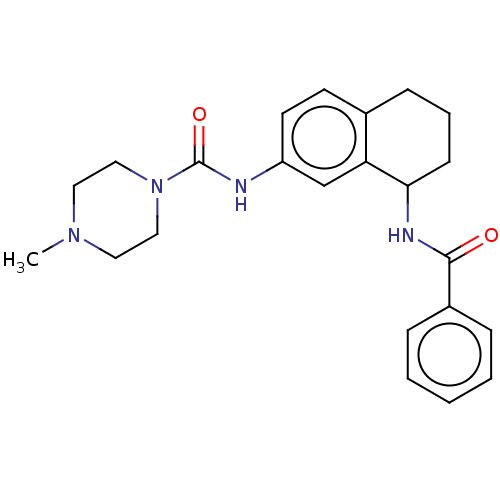
Affinity DataKi: 550nMAssay Description:Activity determined in mouse liver S-adenosyl-L-homocysteine hydrolase and expressed as KI values.More data for this Ligand-Target Pair
Affinity DataKi: 1.04E+3nMAssay Description:Activity determined in mouse liver S-adenosyl-L-homocysteine hydrolase and expressed as KI values.More data for this Ligand-Target Pair
Affinity DataKi: 1.10E+3nMAssay Description:Tested for binding affinity of compound against S-adenosyl-L-homocysteine hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 1.50E+3nMAssay Description:Activity determined in rat liver S-adenosyl-L-homocysteine hydrolase and expressed as Kinactivator values; NA= not applicableMore data for this Ligand-Target Pair
Affinity DataKi: 1.60E+3nMAssay Description:Activity determined in rat liver S-adenosyl-L-homocysteine hydrolase and expressed as Kinactivator values; NA= not applicableMore data for this Ligand-Target Pair
Affinity DataKi: 2.40E+3nMAssay Description:Activity determined in rat liver S-adenosyl-L-homocysteine hydrolase and expressed as KI values.More data for this Ligand-Target Pair
Affinity DataKi: 3.00E+3nMAssay Description:Competitive inhibitory activity against rat liver S-Adenosyl-homocysteine hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 3.20E+3nMAssay Description:Competitive inhibitory activity against rat liver S-Adenosyl-homocysteine hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 6.50E+3nMAssay Description:Activity determined in rat liver S-adenosyl-L-homocysteine hydrolase and expressed as KI values.More data for this Ligand-Target Pair
Affinity DataKi: 6.90E+3nMAssay Description:Tested for binding affinity of compound against S-adenosyl-L-homocysteine hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 9.70E+3nMAssay Description:Competitive inhibitory activity against rat liver S-adenosyl-L-homocysteine hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 9.70E+3nMAssay Description:Activity determined in rat liver S-adenosyl-L-homocysteine hydrolase and expressed as Kinactivator values.More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+4nMAssay Description:Activity determined in mouse liver S-adenosyl-L-homocysteine hydrolase and expressed as KI values.More data for this Ligand-Target Pair
Affinity DataKi: 1.10E+4nMAssay Description:Competitive inhibitory activity against rat liver S-Adenosyl-homocysteine hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 3.00E+4nMAssay Description:Activity determined in mouse liver S-adenosyl-L-homocysteine hydrolase and expressed as KI values.More data for this Ligand-Target Pair
Affinity DataKi: 1.50E+5nMAssay Description:Activity determined in rat liver S-adenosyl-L-homocysteine hydrolase and expressed as Kinactivator values; NA= not applicableMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Inhibition of mouse cathepsin S using benzyloxycarbonyl-L-Leucyl-L-Arginine 4-Methyl-coumaryl-7-amide substrate by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.90nMAssay Description:Inhibition of mouse cathepsin S using benzyloxycarbonyl-L-Leucyl-L-Arginine 4-Methyl-coumaryl-7-amide substrate by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7.70nMAssay Description:Inhibition of human cathepsin S using benzyloxycarbonyl-L-Leucyl-L-Arginine 4-Methyl-coumaryl-7-amide substrate by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibition of human cathepsin S using benzyloxycarbonyl-L-Leucyl-L-Arginine 4-Methyl-coumaryl-7-amide substrate by FRET assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Eli Lilly
Curated by ChEMBL
Eli Lilly
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Eli Lilly
Curated by ChEMBL
Eli Lilly
Curated by ChEMBL
Affinity DataIC50: 22nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Eli Lilly
Curated by ChEMBL
Eli Lilly
Curated by ChEMBL
Affinity DataIC50: 26nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Eli Lilly
Curated by ChEMBL
Eli Lilly
Curated by ChEMBL
Affinity DataIC50: 26nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Eli Lilly
Curated by ChEMBL
Eli Lilly
Curated by ChEMBL
Affinity DataIC50: 47nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Eli Lilly
Curated by ChEMBL
Eli Lilly
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Eli Lilly
Curated by ChEMBL
Eli Lilly
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly
Curated by ChEMBL
Eli Lilly
Curated by ChEMBL
Affinity DataIC50: 53nMAssay Description:Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from hPPARalphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Eli Lilly
Curated by ChEMBL
Eli Lilly
Curated by ChEMBL
Affinity DataIC50: 67nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Eli Lilly
Curated by ChEMBL
Eli Lilly
Curated by ChEMBL
Affinity DataIC50: 72nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Eli Lilly
Curated by ChEMBL
Eli Lilly
Curated by ChEMBL
Affinity DataIC50: 91nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
Affinity DataIC50: 114nMAssay Description:Inhibitory activity against bovine adrenal dopamine beta-hydroxylase(DBH)More data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:Inhibitory activity against bovine adrenal dopamine beta-hydroxylase(DBH)More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly
Curated by ChEMBL
Eli Lilly
Curated by ChEMBL
Affinity DataIC50: 146nMAssay Description:Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from hPPARalphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Eli Lilly
Curated by ChEMBL
Eli Lilly
Curated by ChEMBL
Affinity DataIC50: 161nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
Affinity DataIC50: 210nMAssay Description:Inhibitory activity against bovine adrenal dopamine beta-hydroxylase(DBH)More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly
Curated by ChEMBL
Eli Lilly
Curated by ChEMBL
Affinity DataIC50: 441nMAssay Description:Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from hPPARalphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly
Curated by ChEMBL
Eli Lilly
Curated by ChEMBL
Affinity DataIC50: 449nMAssay Description:Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from hPPARalphaMore data for this Ligand-Target Pair
Affinity DataIC50: 491nMAssay Description:Inhibitory activity against bovine adrenal dopamine beta-hydroxylase(DBH)More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly
Curated by ChEMBL
Eli Lilly
Curated by ChEMBL
Affinity DataIC50: 516nMAssay Description:Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from hPPARalphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Eli Lilly
Curated by ChEMBL
Eli Lilly
Curated by ChEMBL
Affinity DataIC50: 706nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibitory activity against bovine adrenal dopamine beta-hydroxylase(DBH)More data for this Ligand-Target Pair
Affinity DataIC50: 1.17E+3nMAssay Description:Inhibition of human cathepsin S using benzyloxycarbonyl-L-Leucyl-L-Arginine 4-Methyl-coumaryl-7-amide substrate by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.29E+3nMAssay Description:Inhibition of human cathepsin S using benzyloxycarbonyl-L-Leucyl-L-Arginine 4-Methyl-coumaryl-7-amide substrate by FRET assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly
Curated by ChEMBL
Eli Lilly
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from hPPARalphaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.57E+3nMAssay Description:Inhibition of mouse cathepsin S using benzyloxycarbonyl-L-Leucyl-L-Arginine 4-Methyl-coumaryl-7-amide substrate by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibitory activity against bovine adrenal dopamine beta-hydroxylase(DBH)More data for this Ligand-Target Pair
Affinity DataIC50: 2.78E+3nMAssay Description:Inhibition of mouse cathepsin S using benzyloxycarbonyl-L-Leucyl-L-Arginine 4-Methyl-coumaryl-7-amide substrate by FRET assayMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D(Homo sapiens (Human))
Merrell Dow Research Institute
Curated by ChEMBL
Merrell Dow Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.90E+3nMAssay Description:Inhibition of canine heart Phosphodiesterase 4More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly
Curated by ChEMBL
Eli Lilly
Curated by ChEMBL
Affinity DataIC50: 3.39E+3nMAssay Description:Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from hPPARalphaMore data for this Ligand-Target Pair

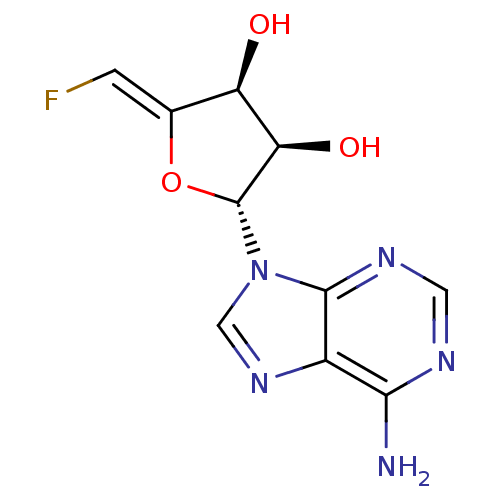

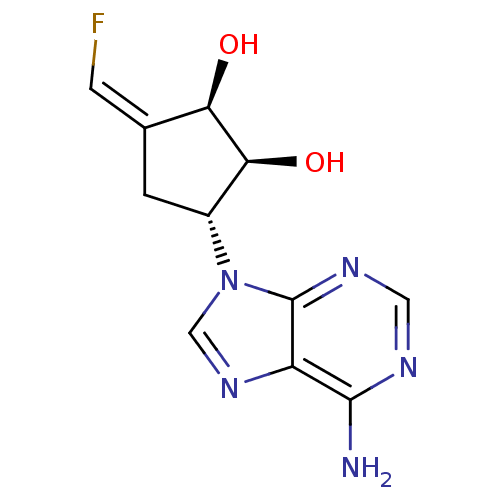

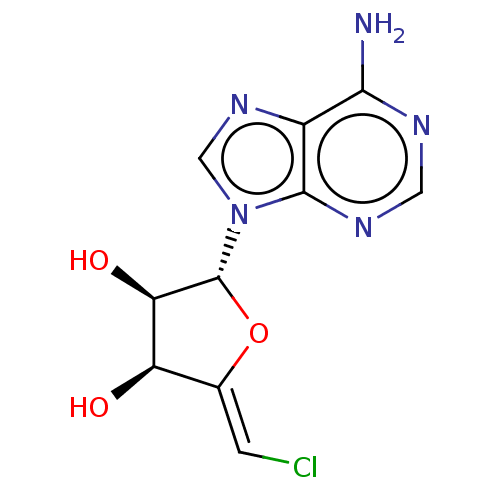

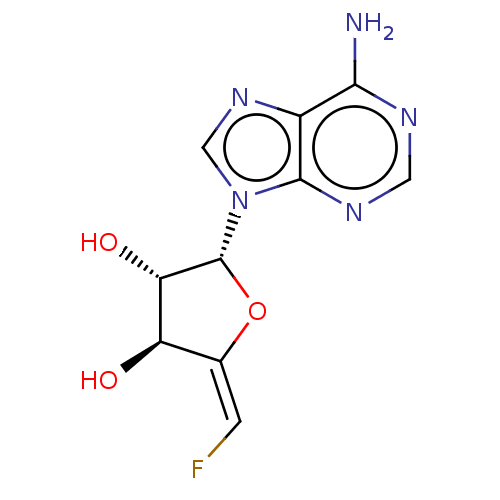
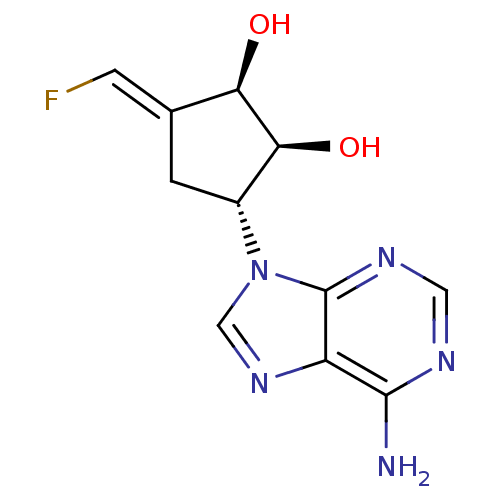



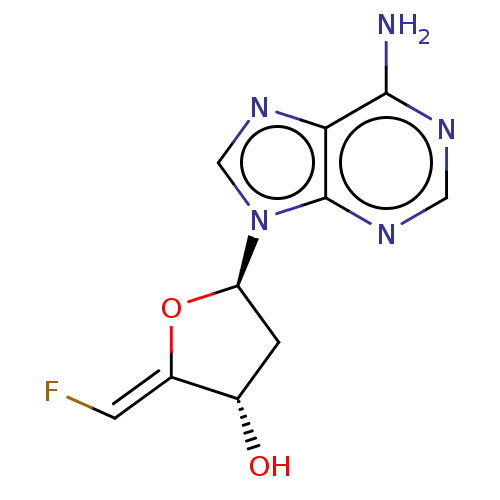

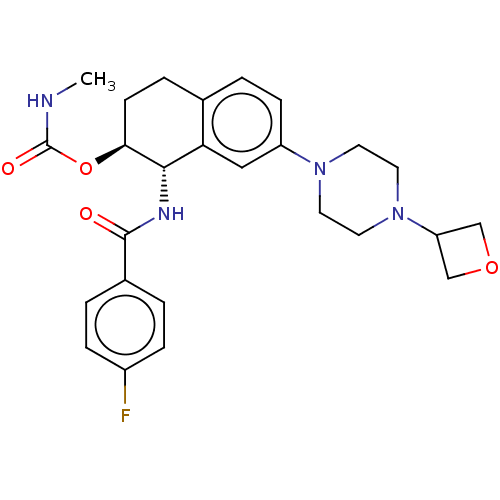
 3D Structure (crystal)
3D Structure (crystal)