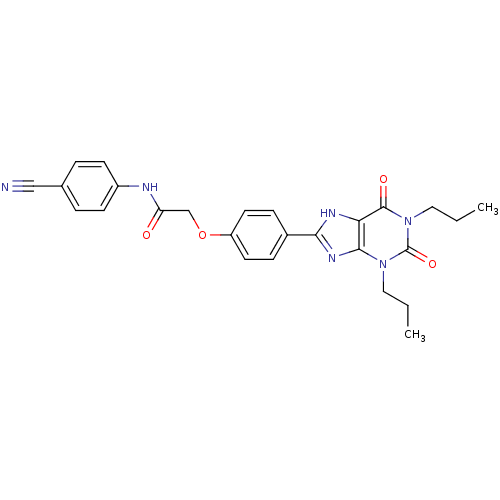
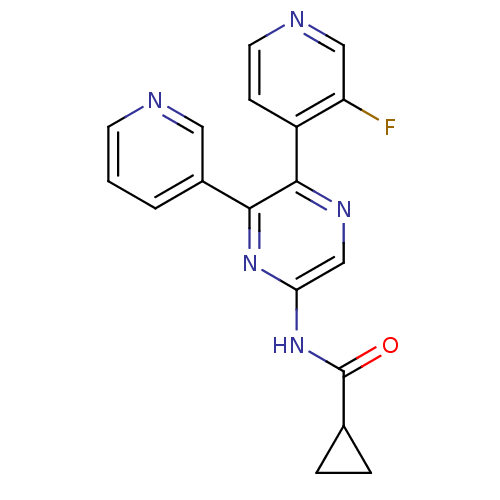
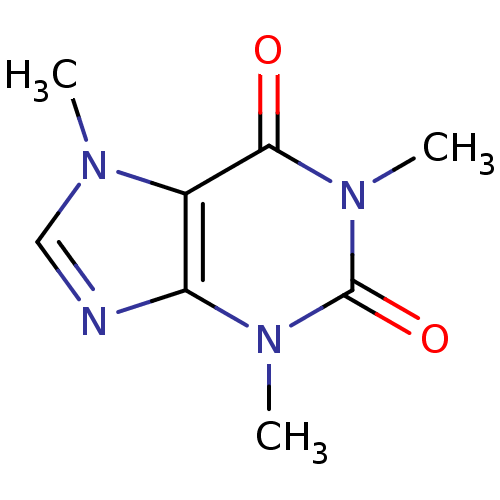
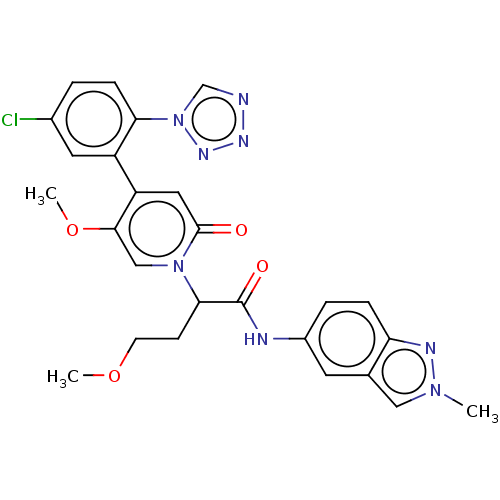
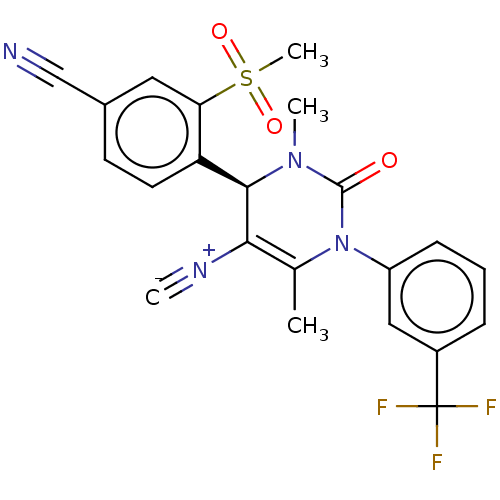
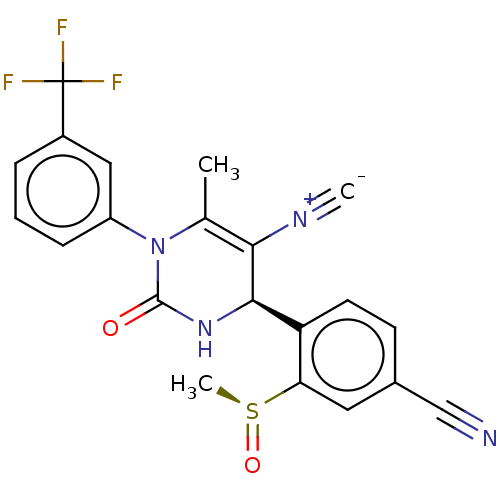
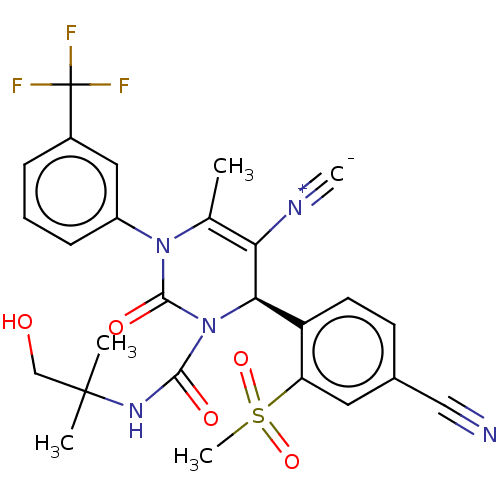
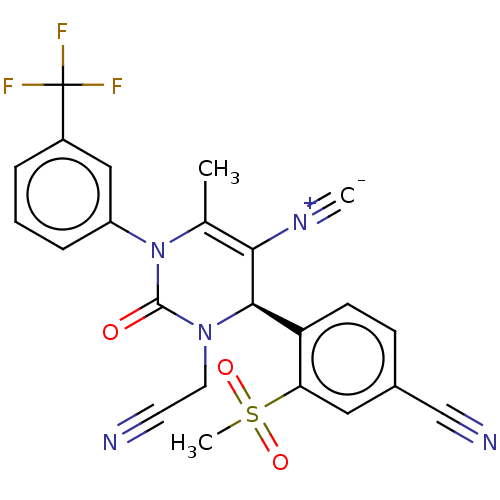
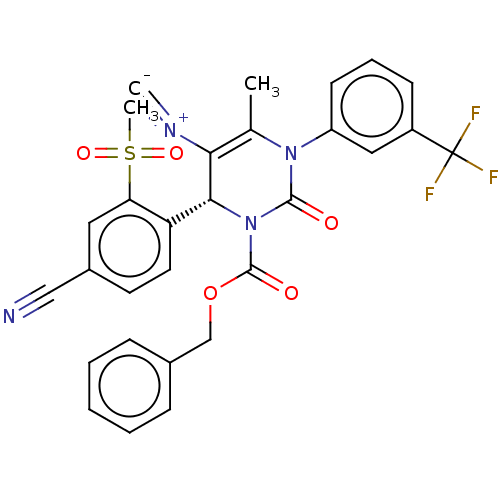
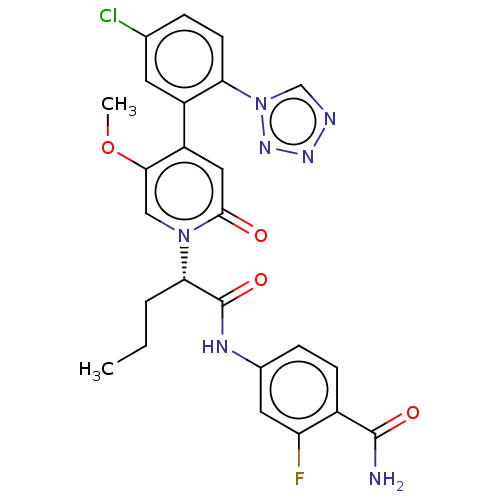
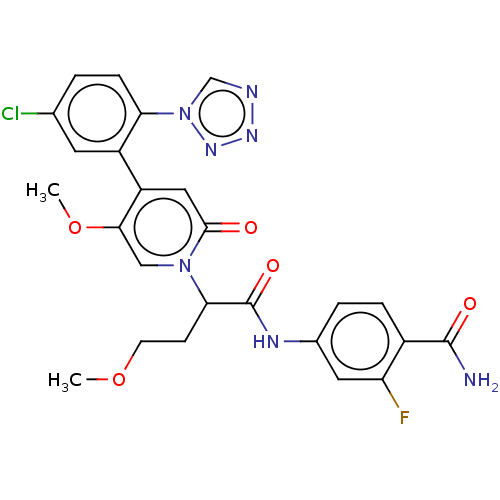
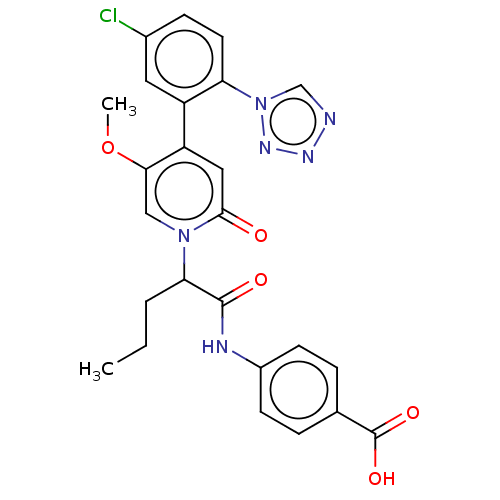
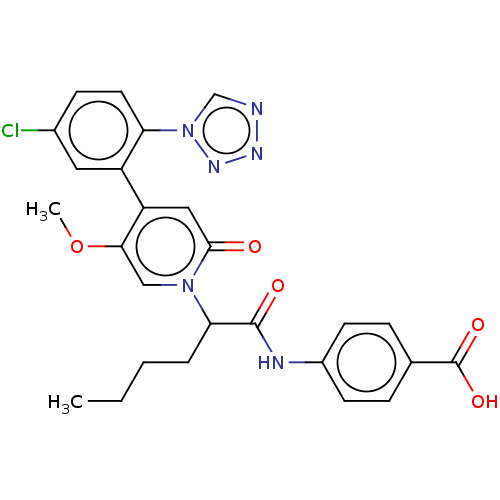
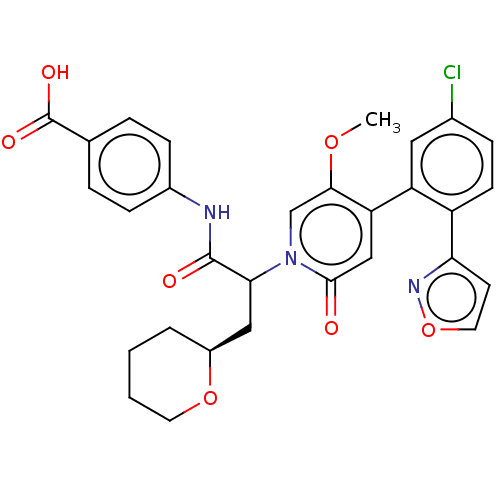
Affinity DataKi: 2nMAssay Description:Inhibition of human A2B adenosine receptorMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Displacement of [3H]PGF2alpha from full-length recombinant human FP receptor expressed in HEK293 cell membranes measured after 60 mins by scintillati...More data for this Ligand-Target Pair
Affinity DataKi: 24nMAssay Description:Inhibition of human A2B adenosine receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]CGS21680 from human A2A adenosine receptor expressed in HEK293 cell membranes after 120 mins by radioligand displacement assayMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in CHO cell membranes after 60 mins by radioligand displacement assayMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]AB-MECA from human A3 adenosine receptor expressed in HEK293 cell membranes after 120 mins by radioligand displacement assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+4nMAssay Description:Inhibition of human A2B adenosine receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.260nMAssay Description:FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin...More data for this Ligand-Target Pair
Affinity DataIC50: 0.260nMAssay Description:FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin...More data for this Ligand-Target Pair
Affinity DataIC50: 0.260nMAssay Description:The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.260nMAssay Description:The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMAssay Description:In vitro HNE inhibition assay. The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amido...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMAssay Description:In vitro HNE inhibition assay. The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amido...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMAssay Description:In vitro HNE inhibition assay. The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amido...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMAssay Description:In vitro HNE inhibition assay. The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amido...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMAssay Description:In vitro HNE inhibition assay. The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amido...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMAssay Description:In vitro HNE inhibition assay. The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amido...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMAssay Description:In vitro HNE inhibition assay. The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amido...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: <0.300nMpH: 7.4 T: 2°CAssay Description:The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept...More data for this Ligand-Target Pair
Affinity DataIC50: 0.310nMAssay Description:FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin...More data for this Ligand-Target Pair
Affinity DataIC50: 0.310nMAssay Description:The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.340nMAssay Description:FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin...More data for this Ligand-Target Pair
Affinity DataIC50: 0.340nMAssay Description:FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin...More data for this Ligand-Target Pair
Affinity DataIC50: 0.340nMAssay Description:The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.340nMAssay Description:The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.360nMAssay Description:FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin...More data for this Ligand-Target Pair
Affinity DataIC50: 0.360nMAssay Description:FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin...More data for this Ligand-Target Pair
Affinity DataIC50: 0.360nMAssay Description:FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin...More data for this Ligand-Target Pair
Affinity DataIC50: 0.360nMAssay Description:The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a...More data for this Ligand-Target Pair