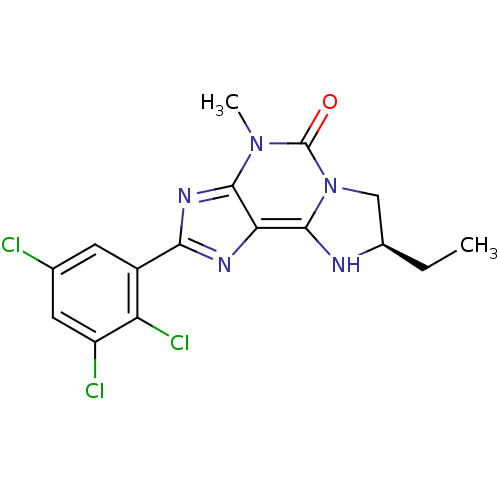
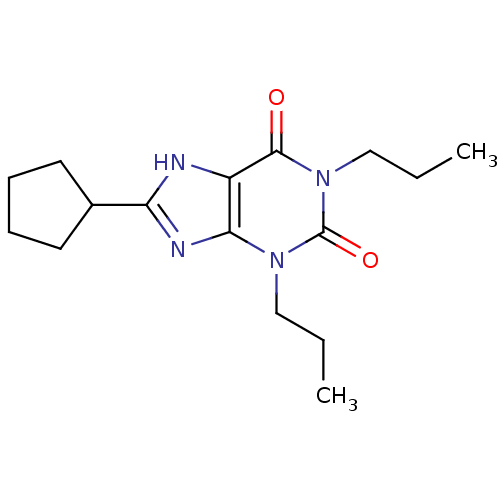
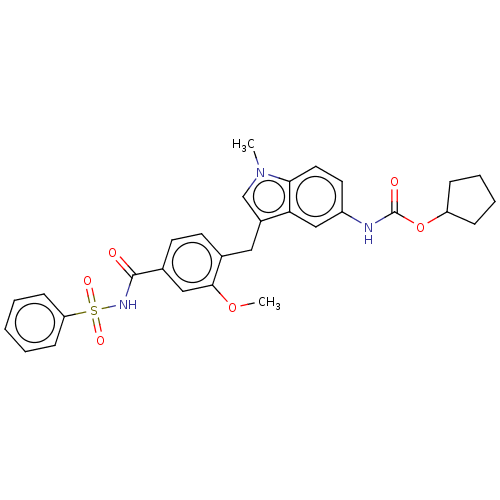
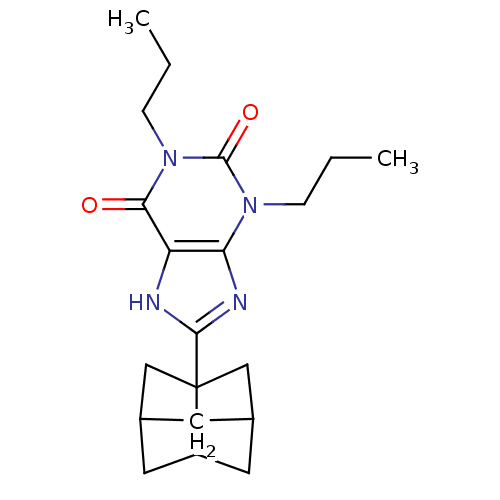
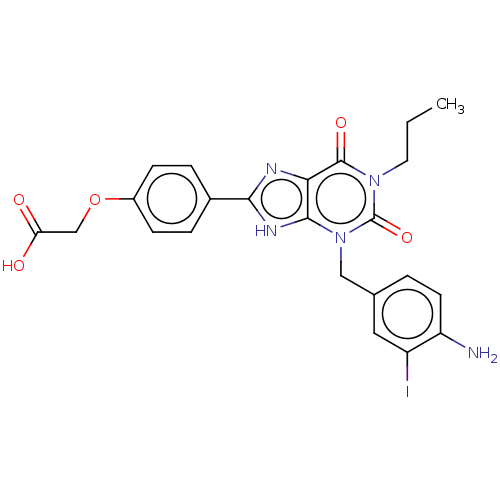
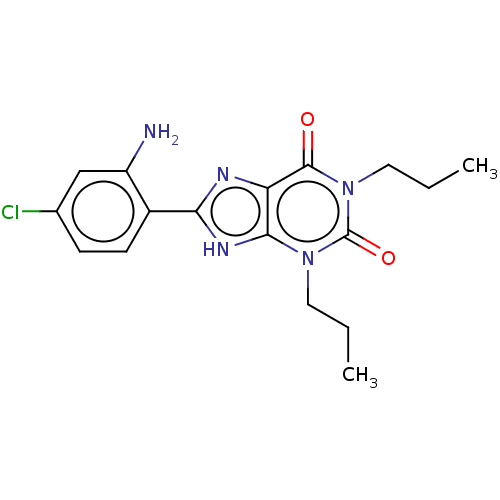
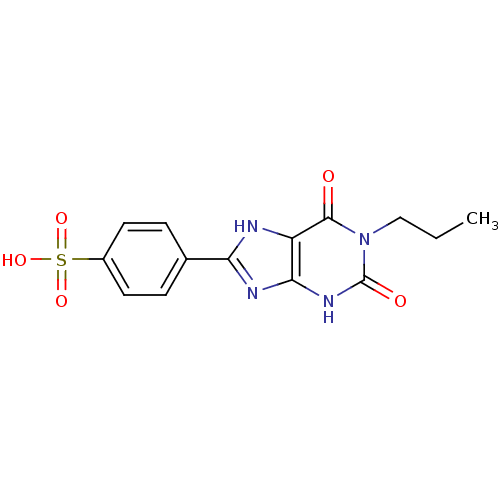
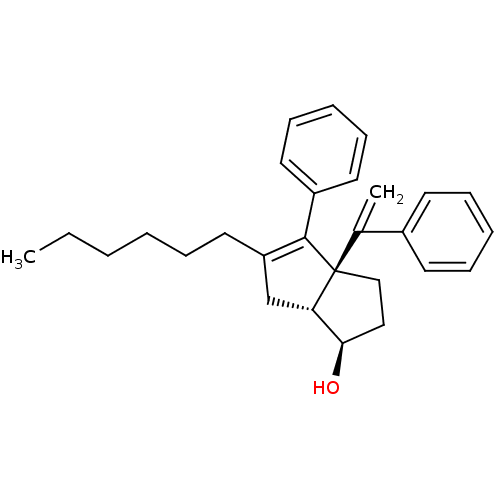
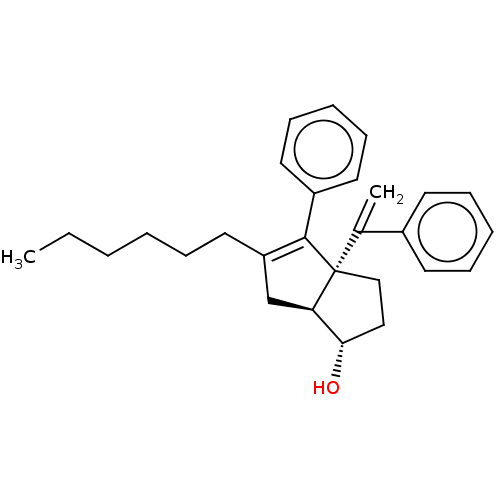
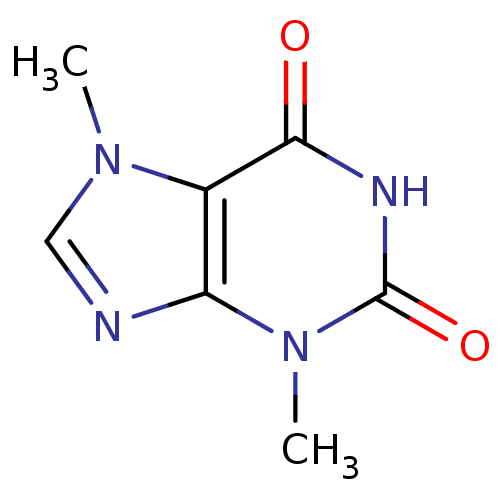
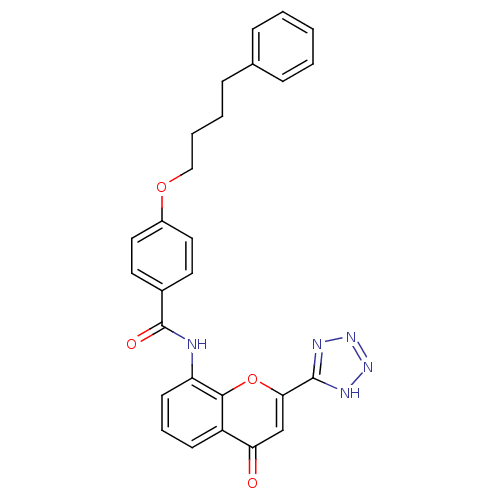
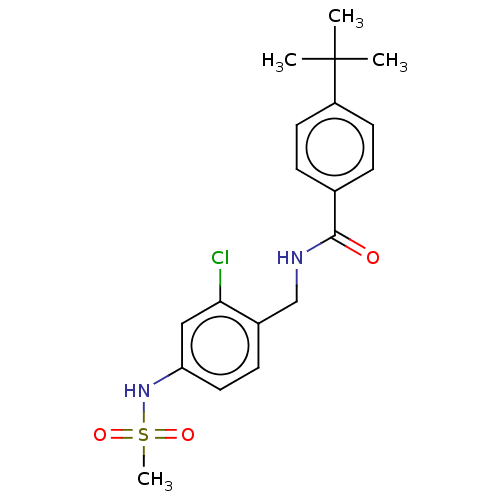
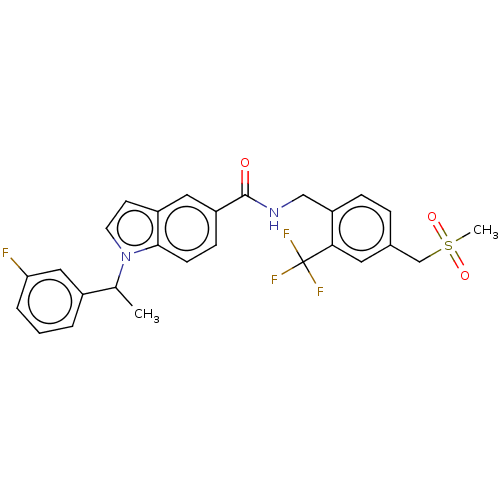
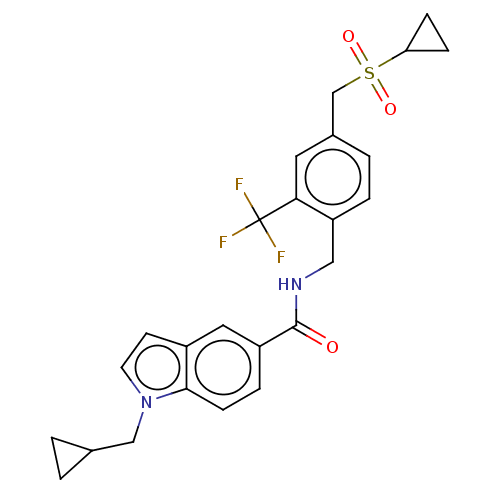
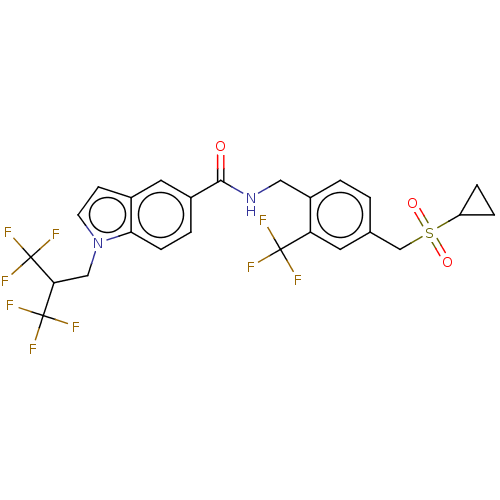
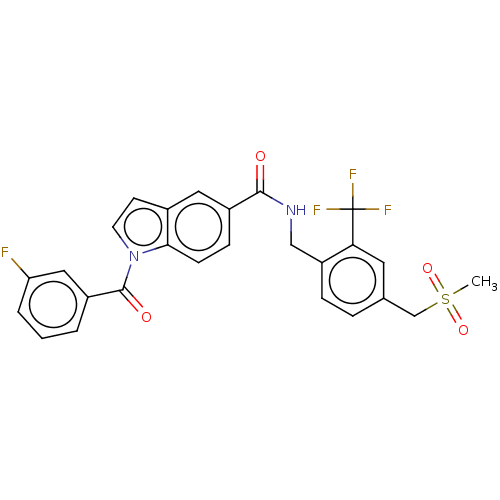
Affinity DataKi: 0.300nMAssay Description:Displacement of [3H]LTD4 from cysteinyl leukotriene receptor 1 in Hartley guinea pig parenchymal membrane after 30 mins by liquid scintillation count...More data for this Ligand-Target Pair
Affinity DataKi: 0.433nMAssay Description:Antagonistic activity of guinea pig Histamine H2 receptor expressed as pA2 at pH 7.4More data for this Ligand-Target Pair
Affinity DataKi: 0.460nMAssay Description:Antagonist activity against beta-1 adrenergic receptor in isolated guinea pig atriaMore data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:Displacement of [3H]LTD4 from cysteinyl leukotriene receptor 1 in Hartley guinea pig parenchymal membrane after 30 mins by liquid scintillation count...More data for this Ligand-Target Pair
Affinity DataKi: 0.950nMAssay Description:Inhibitory activity against dihydrofolate reductase (DHFR) enzyme from Escherichia coliMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1A(Homo sapiens (Human))
Goethe University Frankfurt
Curated by ChEMBL
Goethe University Frankfurt
Curated by ChEMBL
Affinity DataKi: 1.20nMAssay Description:Displacement of [3H]-Way100635 from 5HT1A (unknown origin) expressed in CHO cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:Antagonist activity against beta-1 adrenergic receptor in isolated guinea pig atriaMore data for this Ligand-Target Pair
Affinity DataKi: 1.40nMAssay Description:Antagonistic activity of guinea pig Histamine H2 receptor expressed as pA2 at pH 7.8More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Antagonistic activity against Histamine H2 receptor expressed as the charge of receptor sensitivity was determined at pH 7.8More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Displacement of [3H]-N-methylspiperone from dopamine D3 receptor (unknown origin) expressed in HEK293 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Antagonistic activity against Histamine H2 receptor expressed as the charge of receptor sensitivity was determined at pH 7.0More data for this Ligand-Target Pair
Affinity DataKi: 5.5nMAssay Description:Antagonist activity against beta-1 adrenergic receptor in isolated guinea pig atriaMore data for this Ligand-Target Pair
Affinity DataKi: 6.5nMAssay Description:Displacement of [3H]-N-methylspiperone from dopamine D4 receptor (unknown origin) expressed in HEK293 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 6.5nMAssay Description:Inhibitory activity against dihydrofolate reductase (DHFR) enzyme from Escherichia coliMore data for this Ligand-Target Pair
Affinity DataKi: 6.5nMAssay Description:Antagonist activity against beta-1 adrenergic receptor in isolated guinea pig atriaMore data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated...More data for this Ligand-Target Pair
Affinity DataKi: 22nMAssay Description:Antagonist activity against beta-1 adrenergic receptor in isolated guinea pig atriaMore data for this Ligand-Target Pair
Affinity DataKi: 53nMAssay Description:In vitro inhibitory activity against beta-2 adrenergic receptor was measured by the inhibition of isoproterenol-induced relaxation of PGF2-alpha cont...More data for this Ligand-Target Pair
Affinity DataKi: 54nMAssay Description:Antagonist activity against beta-1 adrenergic receptor in isolated guinea pig atriaMore data for this Ligand-Target Pair
Affinity DataKi: 157nMAssay Description:Displacement of [3H]LTD4 from cysteinyl leukotriene receptor 1 in Hartley guinea pig parenchymal membrane after 30 mins by liquid scintillation count...More data for this Ligand-Target Pair
Affinity DataKi: 229nMAssay Description:In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated...More data for this Ligand-Target Pair
Affinity DataKi: 400nMAssay Description:Binding affinity to human LRH-1More data for this Ligand-Target Pair
Affinity DataKi: 1.20E+3nMAssay Description:Binding affinity to human LRH-1More data for this Ligand-Target Pair
Affinity DataKi: 3.00E+3nMAssay Description:Antagonist activity against beta-1 adrenergic receptor in isolated guinea pig atriaMore data for this Ligand-Target Pair
Affinity DataKi: 4.50E+3nMAssay Description:Antagonist activity against beta-1 adrenergic receptor in isolated guinea pig atriaMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataKi: 4.80E+3nMAssay Description:Competitive inhibition of full length human soluble epoxide hydrolase pre-incubated for 30 mins before DiFMUP substrate addition by fluorescence base...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataKi: 4.80E+3nMAssay Description:Competitive inhibition of full length human soluble epoxide hydrolase pre-incubated for 30 mins before DiFMUP substrate addition by fluorescence base...More data for this Ligand-Target Pair
TargetPlatelet-derived growth factor receptor alpha/beta(Mus musculus (mouse))
J. Uriach & Cía. S.A.
Curated by ChEMBL
J. Uriach & Cía. S.A.
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Inhibitory activity against dihydrofolate reductase (DHFR) enzyme from chicken liverMore data for this Ligand-Target Pair
Affinity DataKi: 1.03E+5nMAssay Description:Inhibitory activity against dihydrofolate reductase (DHFR) enzyme from chicken liverMore data for this Ligand-Target Pair
Affinity DataKi: 1.05E+5nMAssay Description:Ability to inhibit U-46,619-induced contraction of isolated strips of rabbit thoracic aorta, which is the measure of thromboxane receptor antagonismMore data for this Ligand-Target Pair
Affinity DataKi: >2.50E+5nMAssay Description:Ability to inhibit U-46,619-induced contraction of isolated strips of guinea pig tracheal chain, which is the measure of thromboxane receptor antagon...More data for this Ligand-Target Pair
TargetPeptidylglycine alpha-amidating monooxygenase(Rattus norvegicus)
University Of South Florida
Curated by ChEMBL
University Of South Florida
Curated by ChEMBL
Affinity DataKi: 4.70E+5nMAssay Description:Inhibition of rat recombinant peptidylglycine alpha-amidating monooxygenase assessed as inhibition of N-dansyl-Tyr-Val-Gly amidationMore data for this Ligand-Target Pair
TargetPeptidylglycine alpha-amidating monooxygenase(Rattus norvegicus)
University Of South Florida
Curated by ChEMBL
University Of South Florida
Curated by ChEMBL
Affinity DataKi: 3.80E+6nMAssay Description:Inhibition of rat recombinant peptidylglycine alpha-amidating monooxygenase assessed as inhibition of N-dansyl-Tyr-Val-Gly amidationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0440nMAssay Description:Displacement of [3H]ICI from cysteinyl leukotriene receptor 1 in Hartley guinea pig lung membrane after 30 mins by liquid scintillation counting meth...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of sEH in human HepG2 cells using 14(15)-EET-d11 as substrate assessed as reduction in conversion of 14(15)-EET-d11 to 14(15)-DHET-d11 pre...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Antagonist activity at full length human FXR expressed in HeLa cells co-expressing BSEP-pGL3/pSG5-hRXR after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetCysteinyl leukotriene receptor 1(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of cysteinyl leukotriene receptor 1 (unknown origin) expressed in HEK293 cell membranes after 45 mins by scintillation spectrometryMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of recombinant human sEH using PHOME as substrate preincubated for 30 mins followed by substrate addition and measured after 30 mins by fl...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f...More data for this Ligand-Target Pair
TargetCysteinyl leukotriene receptor 1(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 2.30nMAssay Description:Inhibition of cysteinyl leukotriene receptor 1 (unknown origin) expressed in HEK293 cell membranes after 45 mins by scintillation spectrometryMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of recombinant human sEH using PHOME as substrate preincubated for 30 mins followed by substrate addition and measured after 30 mins by fl...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f...More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)