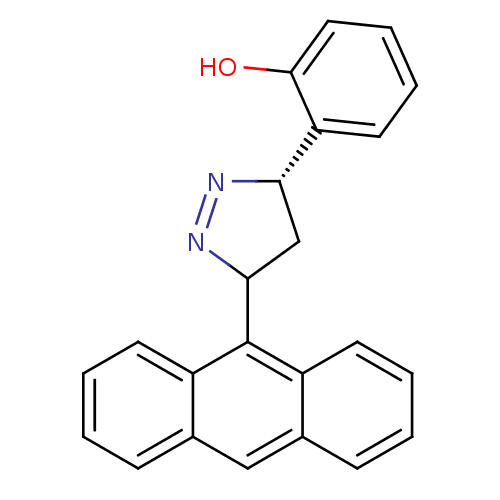
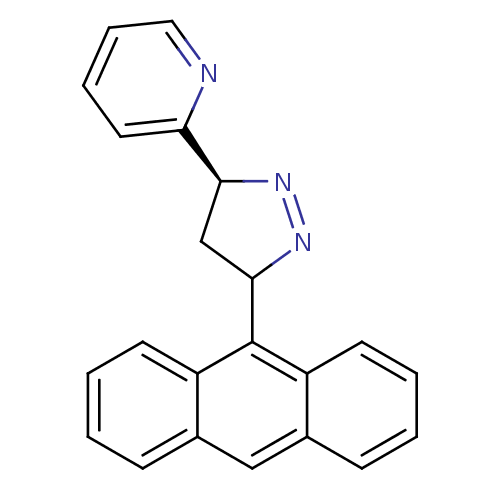
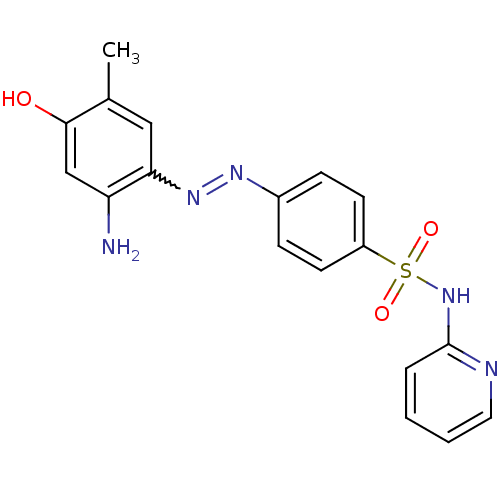
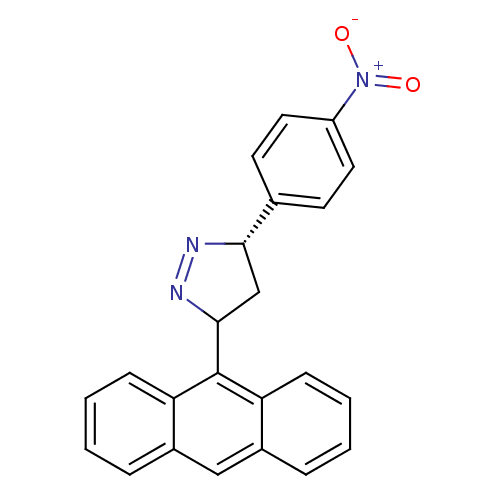
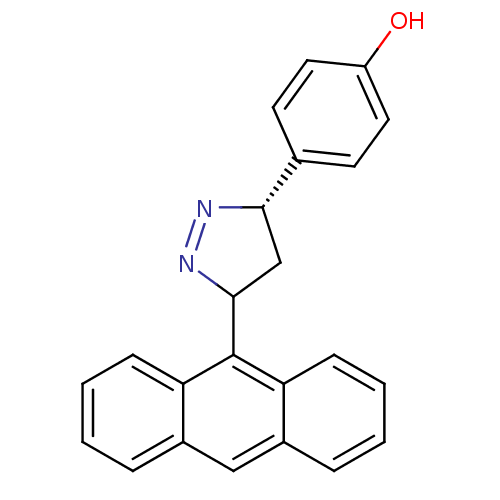
TargetAmine oxidase [flavin-containing] B(Homo sapiens (Human))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 0.310nMAssay Description:Competitive inhibition of human recombinant MAO-B after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Rattus norvegicus (rat))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 0.450nMAssay Description:Competitive inhibition of rat liver MAO-B after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Rattus norvegicus (rat))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 0.600nMAssay Description:Competitive inhibition of rat liver MAO-B after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Homo sapiens (Human))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 1.15nMAssay Description:Competitive inhibition of human recombinant MAO-B after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Homo sapiens (Human))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 1.70nMAssay Description:Competitive inhibition of human recombinant MAO-B after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Homo sapiens (Human))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 1.80nMAssay Description:Competitive inhibition of human recombinant MAO-B after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Rattus norvegicus (rat))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 2.10nMAssay Description:Competitive inhibition of rat liver MAO-B after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Rattus norvegicus (rat))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 2.30nMAssay Description:Competitive inhibition of rat liver MAO-B after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Rattus norvegicus (rat))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 3.43nMAssay Description:Competitive inhibition of rat liver MAO-B after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Homo sapiens (Human))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 3.56nMAssay Description:Competitive inhibition of human recombinant MAO-B after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Rattus norvegicus (rat))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 3.80nMAssay Description:Competitive inhibition of rat liver MAO-B after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Homo sapiens (Human))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 4.21nMAssay Description:Competitive inhibition of human recombinant MAO-B after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Rattus norvegicus (rat))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 4.77nMAssay Description:Competitive inhibition of rat liver MAO-B after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Homo sapiens (Human))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 5.54nMAssay Description:Competitive inhibition of human recombinant MAO-B after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Homo sapiens (Human))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 5.75nMAssay Description:Competitive inhibition of human recombinant MAO-B after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Rattus norvegicus (rat))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 5.91nMAssay Description:Competitive inhibition of rat liver MAO-B after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Rattus norvegicus (rat))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 6.75nMAssay Description:Competitive inhibition of rat liver MAO-B after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataKi: 7.20nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataKi: 7.20nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's methodMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Homo sapiens (Human))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 7.35nMAssay Description:Competitive inhibition of human recombinant MAO-B after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Homo sapiens (Human))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 7.51nMAssay Description:Competitive inhibition of human recombinant MAO-B after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Rattus norvegicus (rat))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 9.91nMAssay Description:Competitive inhibition of rat liver MAO-B after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 17.1nMAssay Description:Competitive inhibition of human recombinant MAO-A after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Rattus norvegicus (rat))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 20.1nMAssay Description:Competitive inhibition of rat liver MAO-A after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataKi: 21nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataKi: 24nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's methodMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 32.2nMAssay Description:Competitive inhibition of human recombinant MAO-A after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataKi: 41nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's methodMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Rattus norvegicus (rat))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 43.9nMAssay Description:Competitive inhibition of rat liver MAO-A after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 69.9nMAssay Description:Competitive inhibition of human recombinant MAO-A after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Rattus norvegicus (rat))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 77.6nMAssay Description:Competitive inhibition of rat liver MAO-A after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 81.5nMAssay Description:Competitive inhibition of human recombinant MAO-A after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetBromodomain-containing protein 4(Homo sapiens (Human))
University Of Minnesota
Curated by ChEMBL
University Of Minnesota
Curated by ChEMBL
Affinity DataKi: <85nMAssay Description:Inhibition of human 6x-His-tagged BRD4 bromodomain 1 expressed in Escherichia coliMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Rattus norvegicus (rat))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 95.7nMAssay Description:Competitive inhibition of rat liver MAO-A after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataKi: >100nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataKi: >100nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's methodMore data for this Ligand-Target Pair
TargetBromodomain-containing protein 3(Homo sapiens (Human))
University Of Minnesota
Curated by ChEMBL
University Of Minnesota
Curated by ChEMBL
Affinity DataKi: 100nMAssay Description:Inhibition of human 6x-His-tagged BRD3 bromodomain 1 expressed in Escherichia coliMore data for this Ligand-Target Pair
Affinity DataKi: >100nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's methodMore data for this Ligand-Target Pair
TargetBromodomain-containing protein 3(Homo sapiens (Human))
University Of Minnesota
Curated by ChEMBL
University Of Minnesota
Curated by ChEMBL
Affinity DataKi: 140nMAssay Description:Inhibition of human 6x-His-tagged BRD3 bromodomain 2 expressed in Escherichia coliMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 205nMAssay Description:Competitive inhibition of human recombinant MAO-A after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 225nMAssay Description:Competitive inhibition of human recombinant MAO-A after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Rattus norvegicus (rat))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 230nMAssay Description:Competitive inhibition of rat liver MAO-A after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Rattus norvegicus (rat))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 242nMAssay Description:Competitive inhibition of rat liver MAO-A after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 250nMAssay Description:Competitive inhibition of human recombinant MAO-A after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 281nMAssay Description:Competitive inhibition of human recombinant MAO-A after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 301nMAssay Description:Competitive inhibition of human recombinant MAO-A after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Rattus norvegicus (rat))
Birla Institute Of Technology
Curated by ChEMBL
Birla Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 320nMAssay Description:Competitive inhibition of rat liver MAO-A after 60 mins using p-tyramine as substrate by spectrophotometryMore data for this Ligand-Target Pair

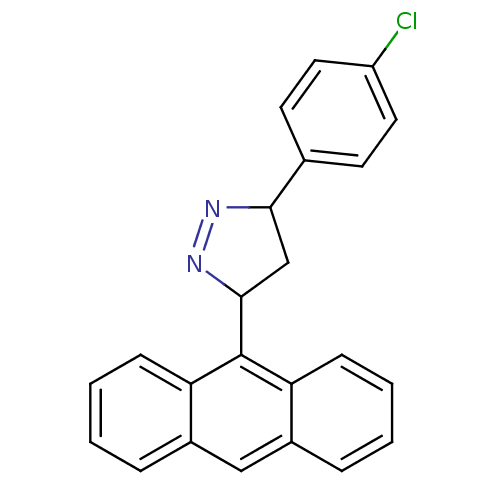

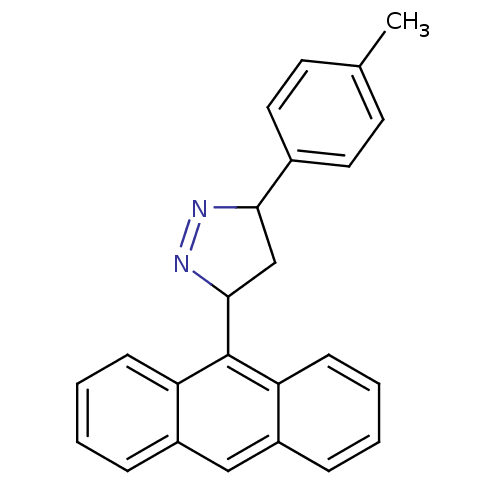
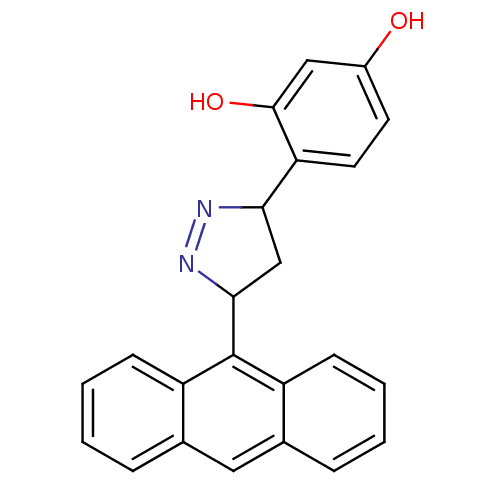
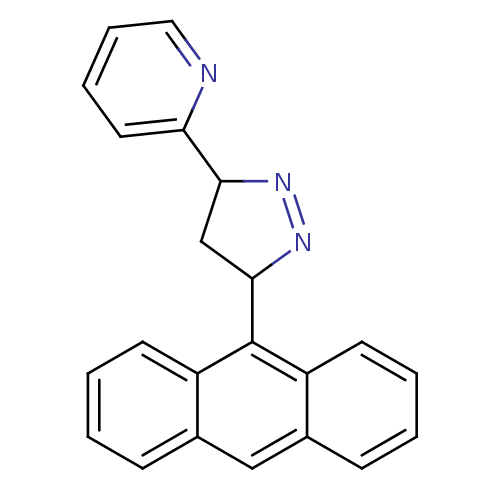

 3D Structure (crystal)
3D Structure (crystal)