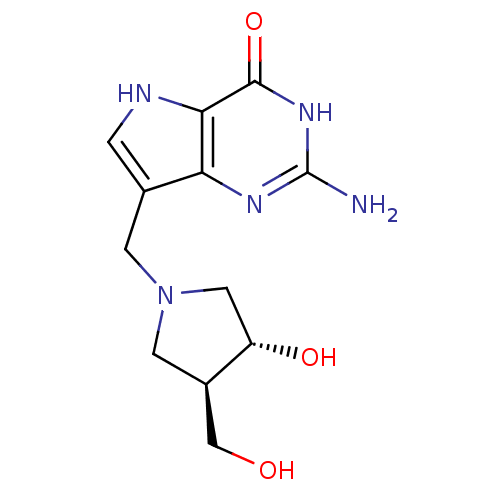
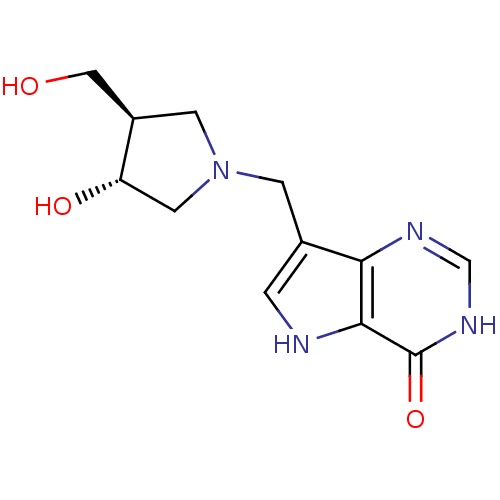
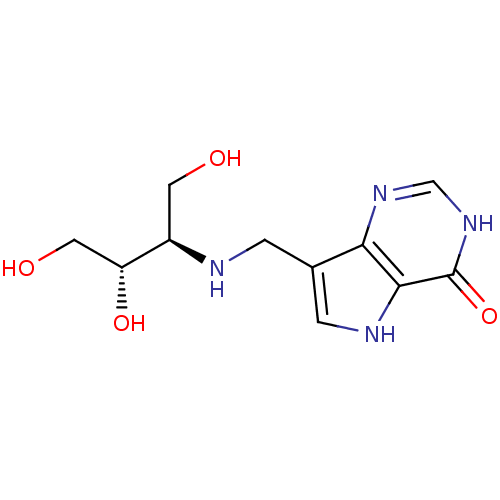
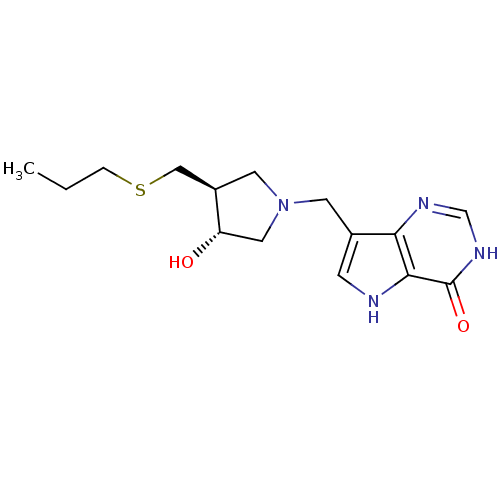
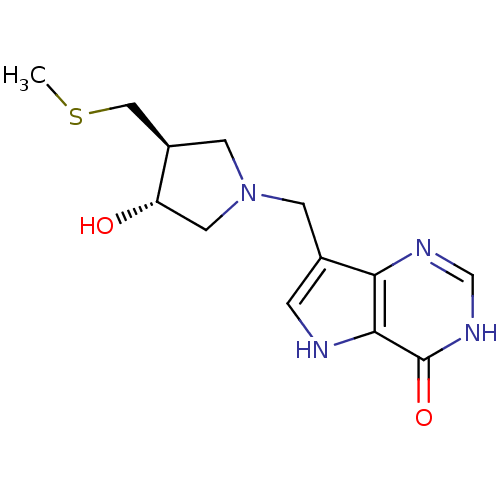
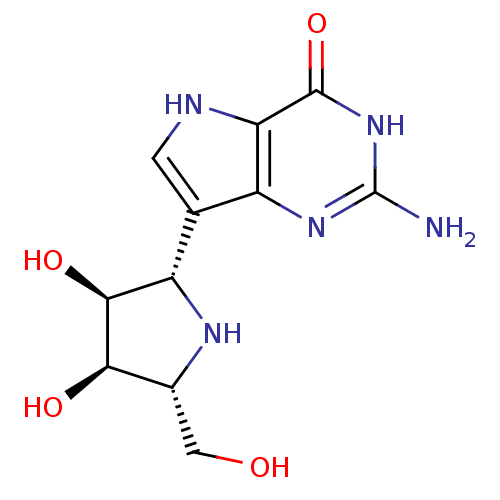
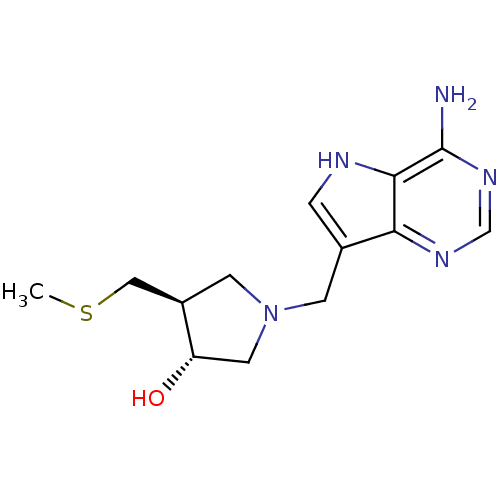
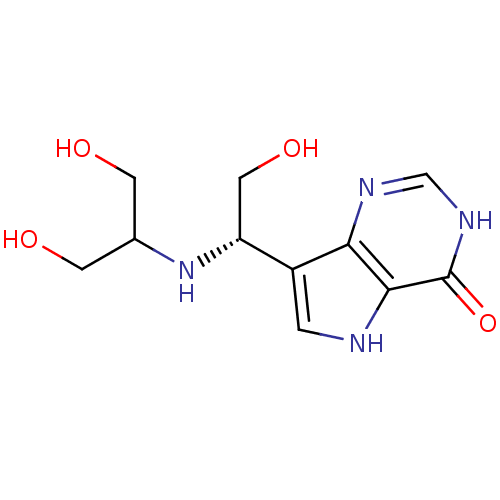
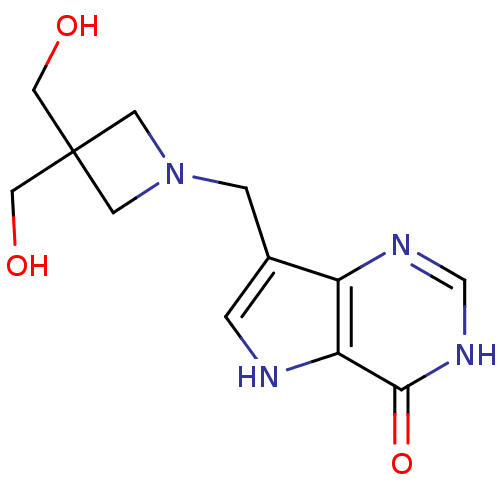
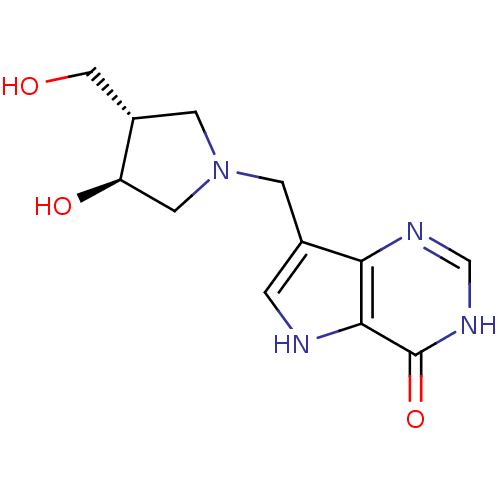
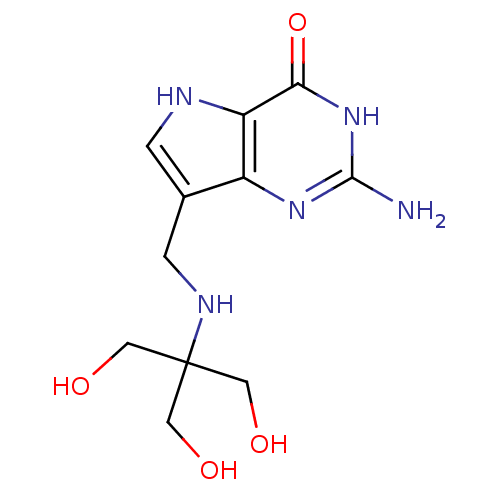
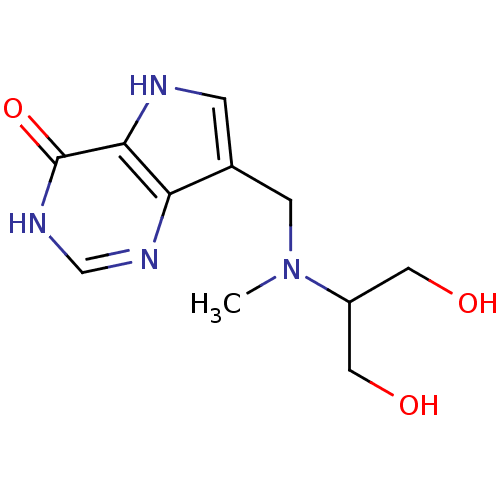
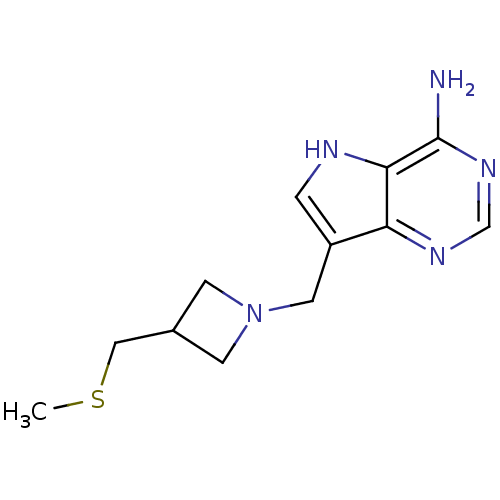
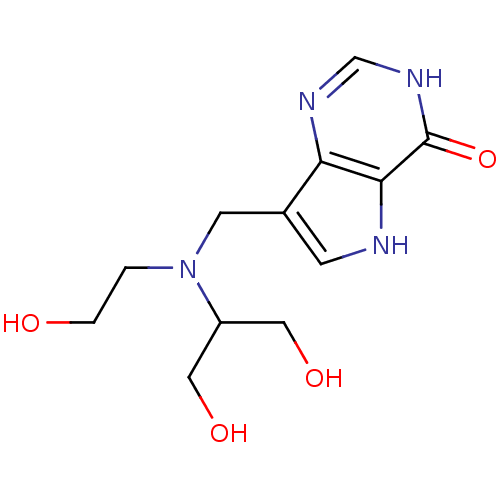
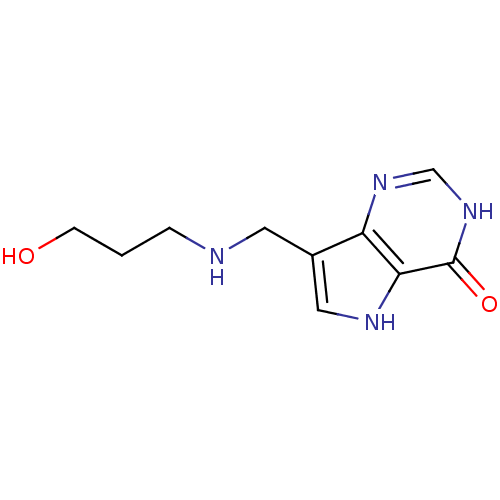
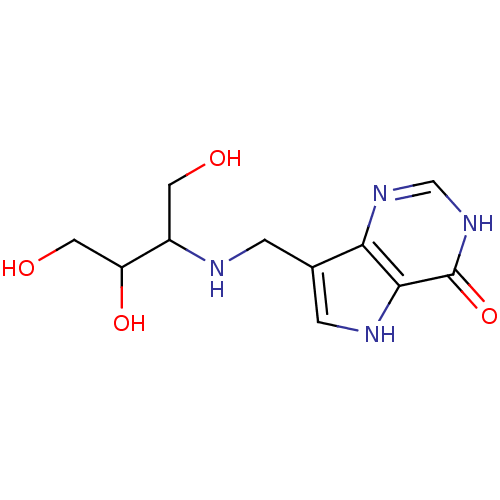
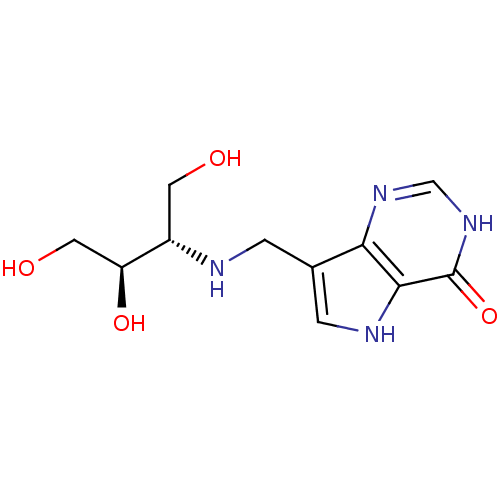
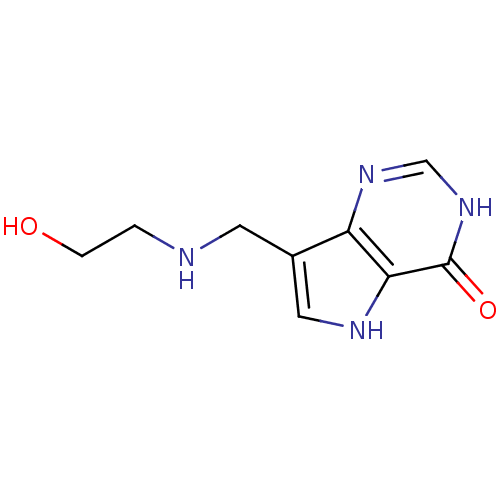
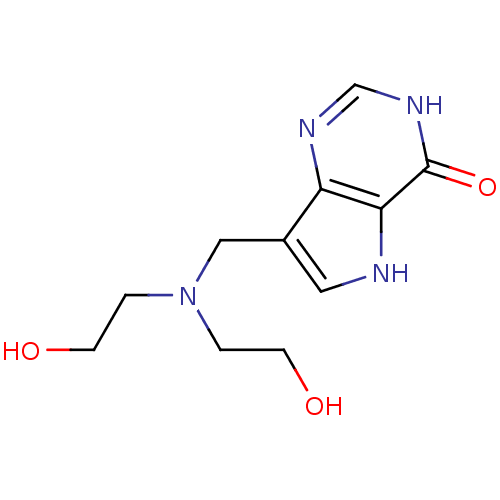
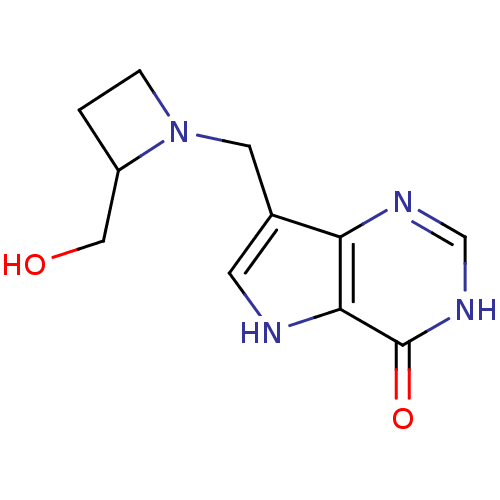
Affinity DataKi: 0.00200nMAssay Description:Inhibition of human PNP by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.00300nMAssay Description:Inhibition of human PNP by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.00500nMAssay Description:Inhibition of human PNP by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.00520nMAssay Description:Equilibrium binding affinity to wild type human PNPMore data for this Ligand-Target Pair
Affinity DataKi: 0.00700nMAssay Description:Inhibition of human PNP by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.00900nMAssay Description:Inhibition of human PNP by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.00900nMAssay Description:Inhibition of human PNP by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.00980nMAssay Description:Equilibrium binding affinity to wild type human PNPMore data for this Ligand-Target Pair
Affinity DataKi: 0.0107nMAssay Description:Equilibrium binding affinity to wild type human PNPMore data for this Ligand-Target Pair
Affinity DataKi: 0.0110nMAssay Description:Inhibition of human purine nucleoside phosphorylase by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.0196nMAssay Description:Equilibrium binding affinity to wild type human PNPMore data for this Ligand-Target Pair
Affinity DataKi: 0.0420nMAssay Description:Inhibition of human PNP by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Target5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase(Escherichia coli (strain K12))
Industrial Research
Industrial Research
Affinity DataKi: 0.0480nM ΔG°: -58.3kJ/molepH: 7.0 T: 2°CAssay Description:Enzyme activity was monitored by absorbance change in the xanthine oxidase coupled assay, which measures the formation of 2,8-dihydroxyadenine at 293...More data for this Ligand-Target Pair
Affinity DataKi: 0.0560nMAssay Description:Inhibition of human PNP by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.0579nMAssay Description:Equilibrium binding affinity to wild type human PNPMore data for this Ligand-Target Pair
Affinity DataKi: 0.0660nMAssay Description:Inhibition of human purine nucleoside phosphorylase by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.0660nMAssay Description:Inhibition of human purine nucleoside phosphorylase by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.100nMAssay Description:Initial binding affinity to wild type human PNPMore data for this Ligand-Target Pair
Affinity DataKi: 0.110nMAssay Description:Initial binding affinity to wild type human PNPMore data for this Ligand-Target Pair
Affinity DataKi: 0.117nMAssay Description:Initial binding affinity to wild type human PNPMore data for this Ligand-Target Pair
Affinity DataKi: 0.210nMAssay Description:Inhibition of human PNP by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.229nM ΔG°: -54.5kJ/molepH: 7.7 T: 2°CAssay Description:PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co...More data for this Ligand-Target Pair
Affinity DataKi: 0.229nMAssay Description:Inhibition of human PNP by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.380nMAssay Description:Inhibition of human PNP by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.380nMAssay Description:Inhibition of human purine nucleoside phosphorylase assessed as slow onset inhibition constant by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Inhibition of human PNP by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.430nMAssay Description:Inhibition of human PNP by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Target5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase(Escherichia coli (strain K12))
Industrial Research
Industrial Research
Affinity DataKi: 0.450nM ΔG°: -52.8kJ/molepH: 7.0 T: 2°CAssay Description:Enzyme activity was monitored by absorbance change in the xanthine oxidase coupled assay, which measures the formation of 2,8-dihydroxyadenine at 293...More data for this Ligand-Target Pair
Affinity DataKi: 0.469nMAssay Description:Inhibition of human PNP by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nM ΔG°: -52.6kJ/molepH: 7.7 T: 2°CAssay Description:PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Inhibition of human purine nucleoside phosphorylase assessed as slow onset inhibition constant by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Inhibition of Plasmodium falciparum purine nucleoside phosphorylase assessed as slow onset inhibition constant by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Inhibition of human purine nucleoside phosphorylase assessed as slow onset inhibition constant by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.620nMAssay Description:Inhibition of human PNP by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.665nM ΔG°: -51.9kJ/molepH: 7.7 T: 2°CAssay Description:PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co...More data for this Ligand-Target Pair
Affinity DataKi: 0.780nMAssay Description:Inhibition of human PNP by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.780nMAssay Description:Inhibition of human PNP by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Target5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase(Escherichia coli (strain K12))
Industrial Research
Industrial Research
Affinity DataKi: 0.840nM ΔG°: -51.3kJ/molepH: 7.0 T: 2°CAssay Description:Enzyme activity was monitored by absorbance change in the xanthine oxidase coupled assay, which measures the formation of 2,8-dihydroxyadenine at 293...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Inhibition of human PNP by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 1nM ΔG°: -50.9kJ/molepH: 7.7 T: 2°CAssay Description:PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co...More data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Inhibition of human purine nucleoside phosphorylase assessed as slow onset inhibition constant by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Initial binding affinity to wild type human PNPMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Inhibition of human PNP by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nM ΔG°: -50.6kJ/molepH: 7.7 T: 2°CAssay Description:PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co...More data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:Inhibition of human PNP by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.70nM ΔG°: -49.6kJ/molepH: 7.0 T: 2°CAssay Description:Enzyme activity was monitored by absorbance change in the xanthine oxidase coupled assay, which measures the formation of 2,8-dihydroxyadenine at 293...More data for this Ligand-Target Pair
Affinity DataKi: 1.80nM ΔG°: -49.4kJ/molepH: 7.7 T: 2°CAssay Description:PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co...More data for this Ligand-Target Pair
Affinity DataKi: 1.80nM ΔG°: -49.4kJ/molepH: 7.7 T: 2°CAssay Description:PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co...More data for this Ligand-Target Pair
Affinity DataKi: 1.82nMAssay Description:Inhibition of human purine nucleoside phosphorylase by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 2nM ΔG°: -49.2kJ/molepH: 7.0 T: 2°CAssay Description:Enzyme activity was monitored by absorbance change in the xanthine oxidase coupled assay, which measures the formation of 2,8-dihydroxyadenine at 293...More data for this Ligand-Target Pair

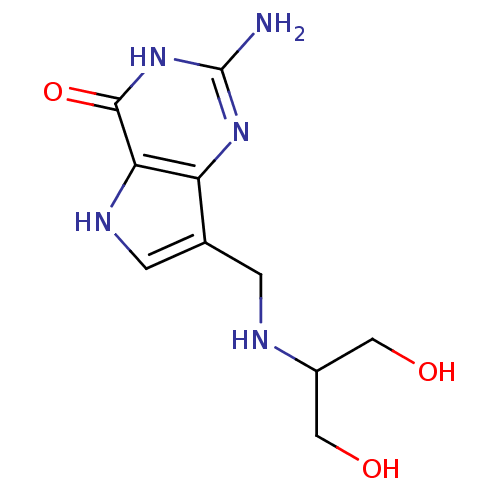
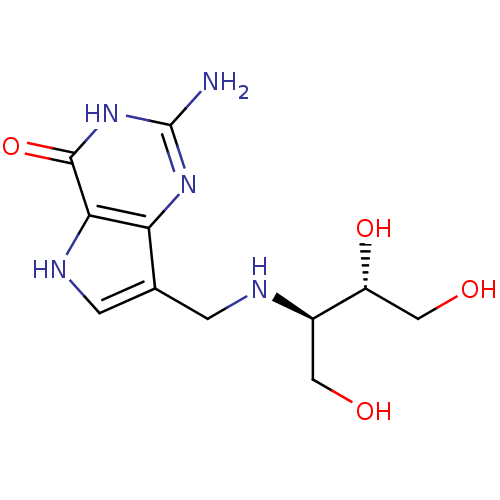

 3D Structure (crystal)
3D Structure (crystal)