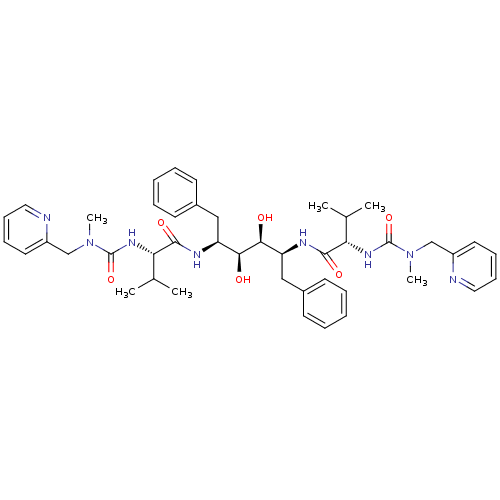
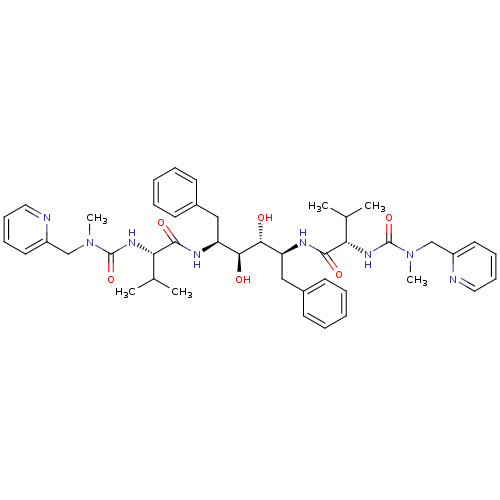
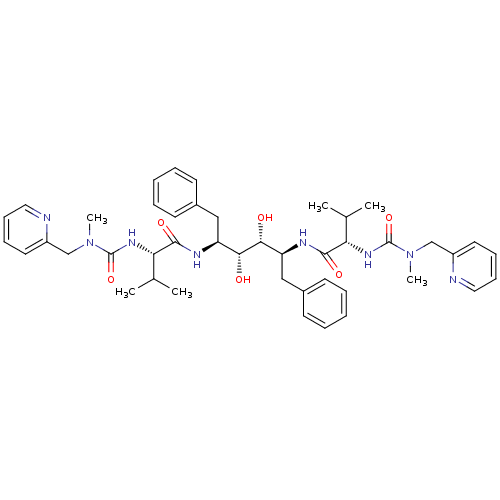
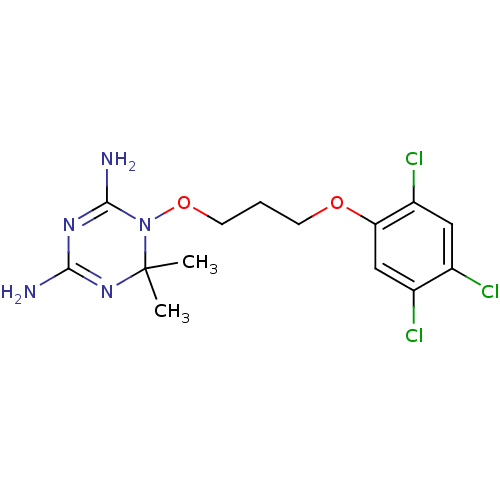
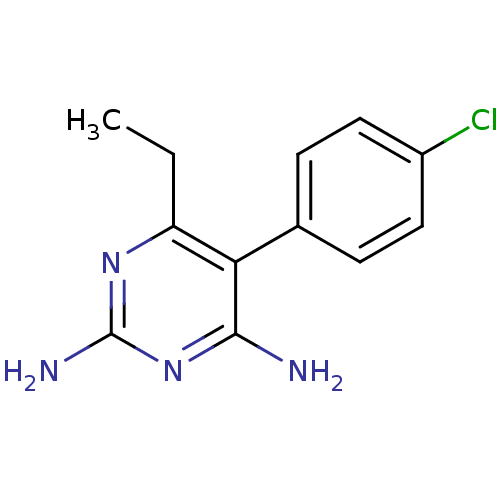
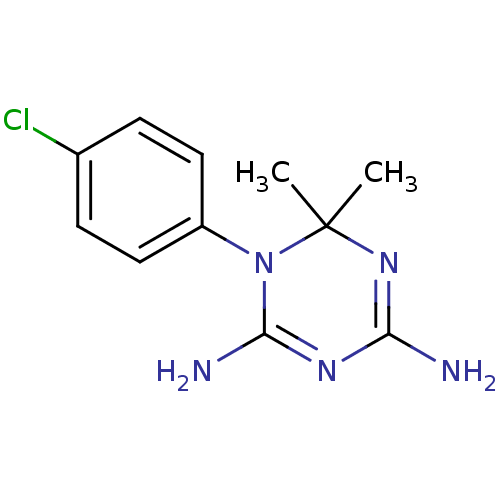
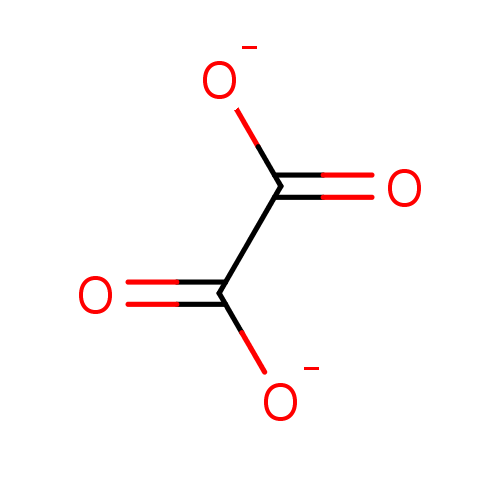
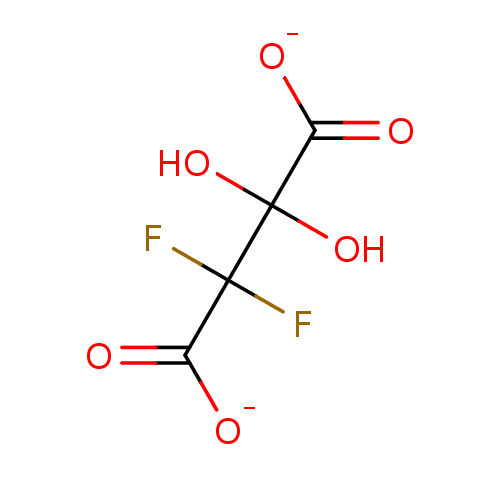
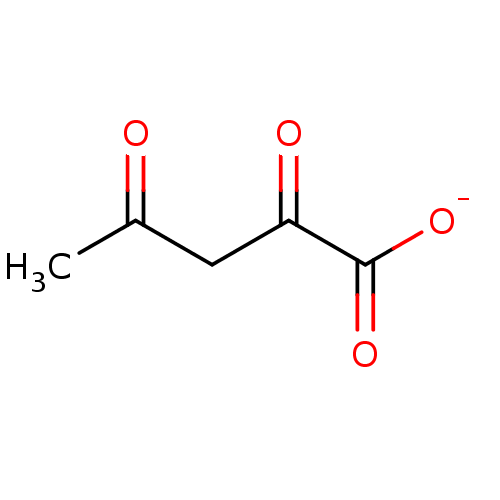
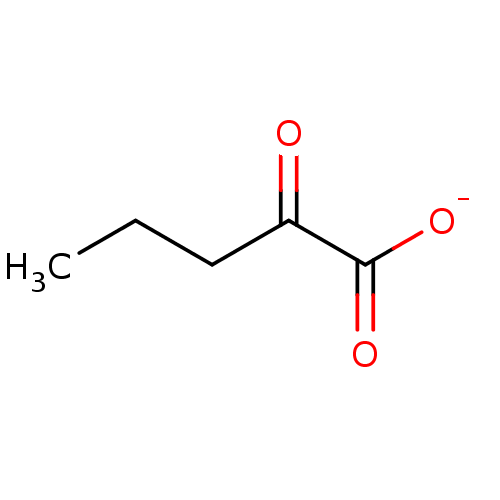
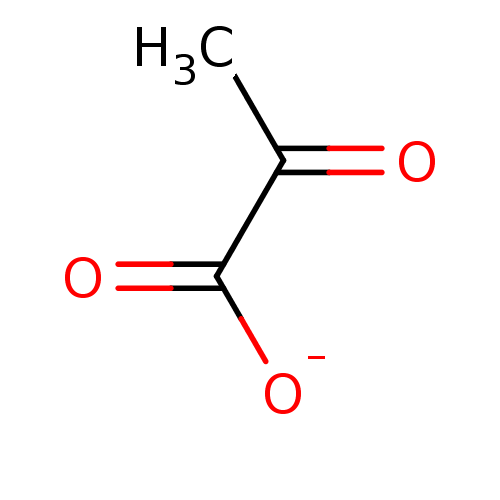
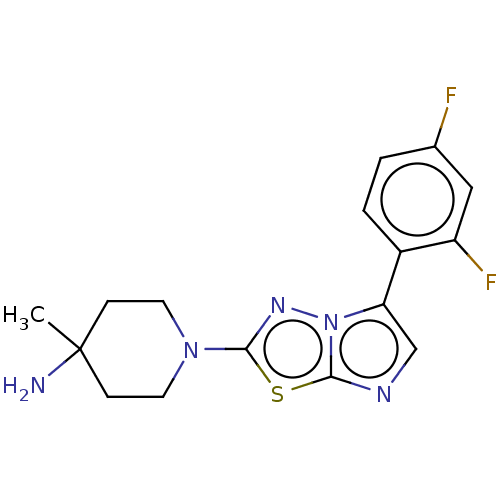
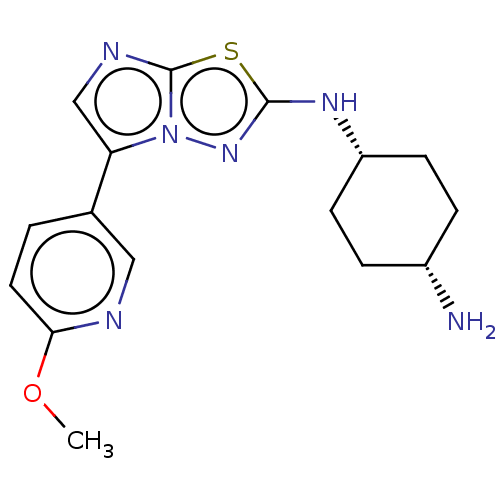
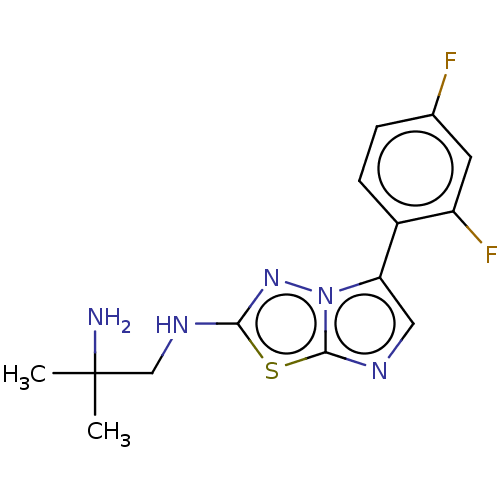
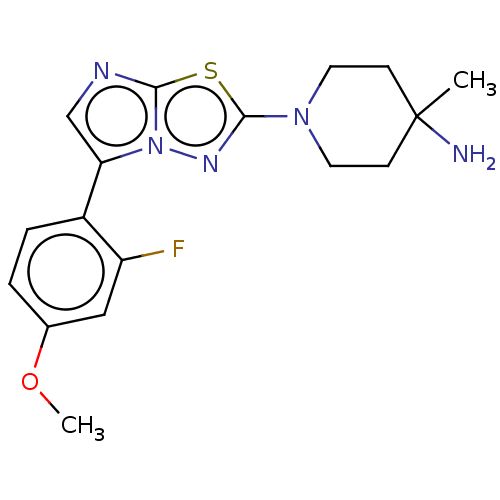
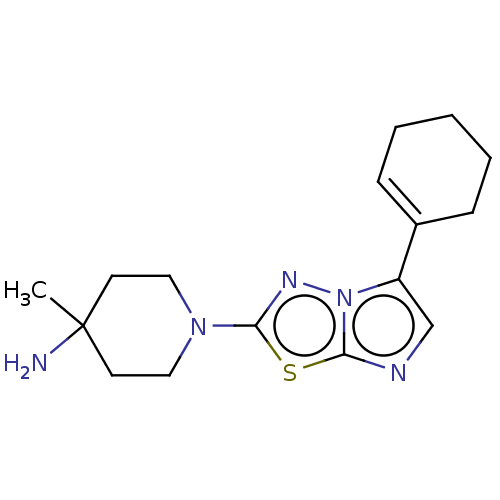
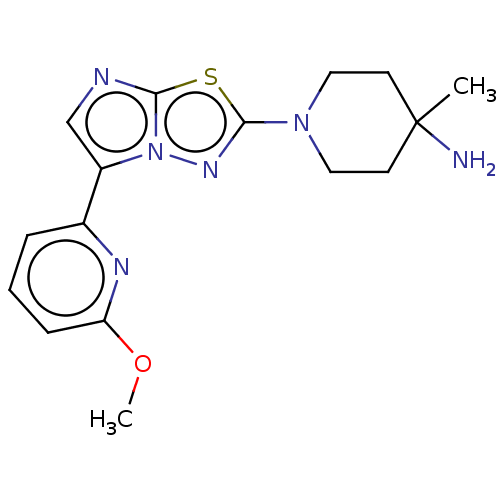
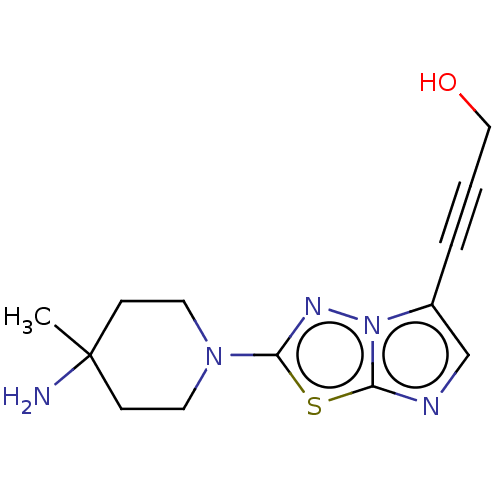
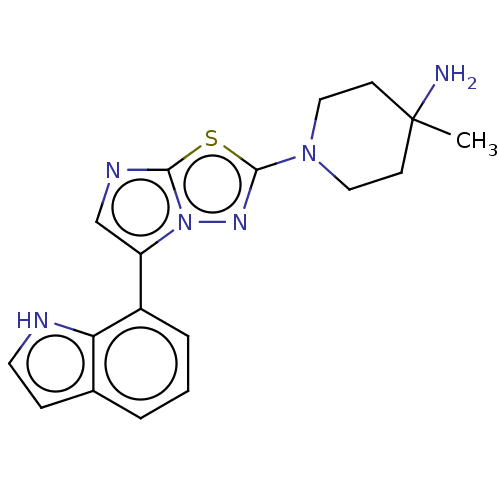
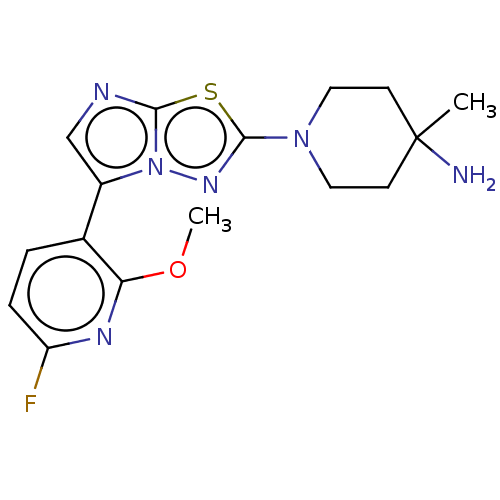
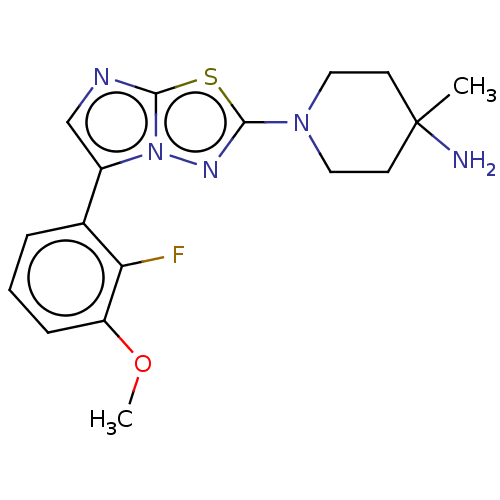
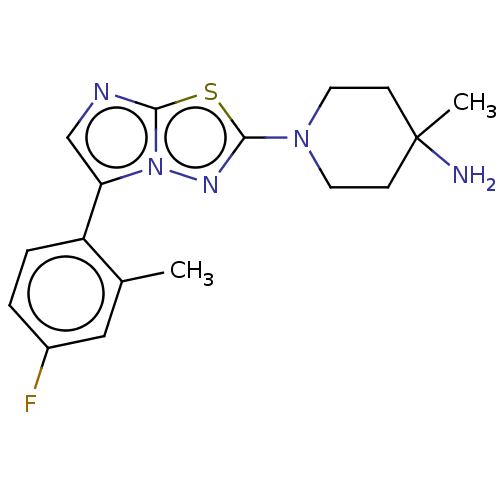
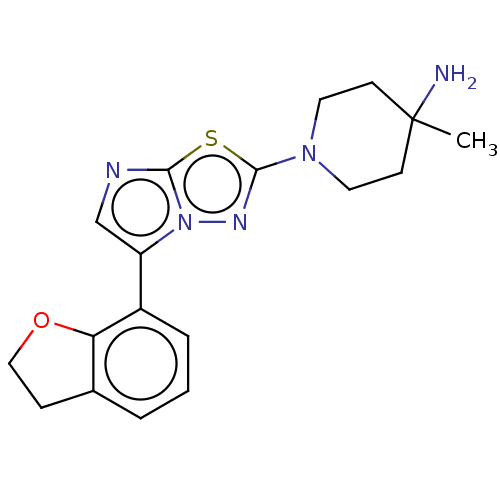
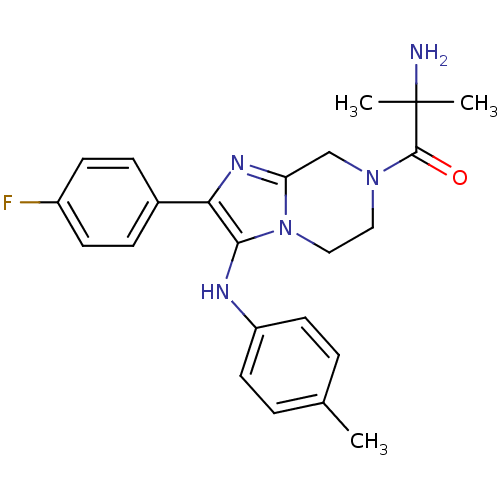
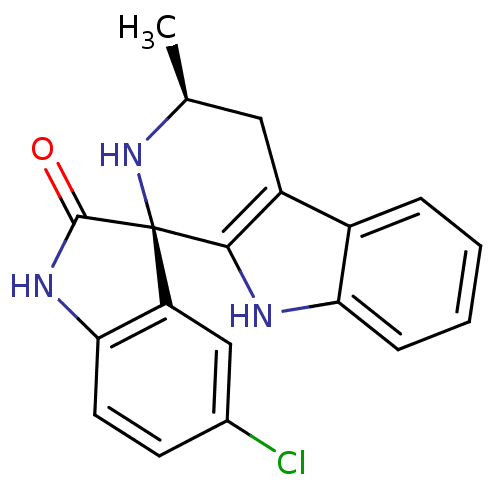
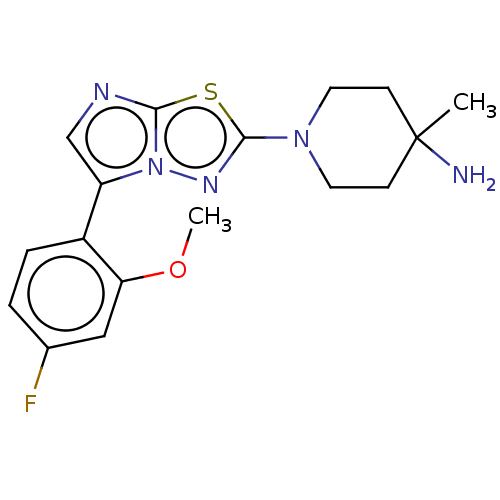
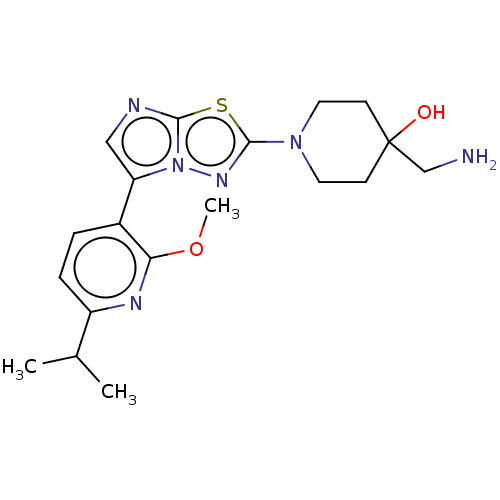
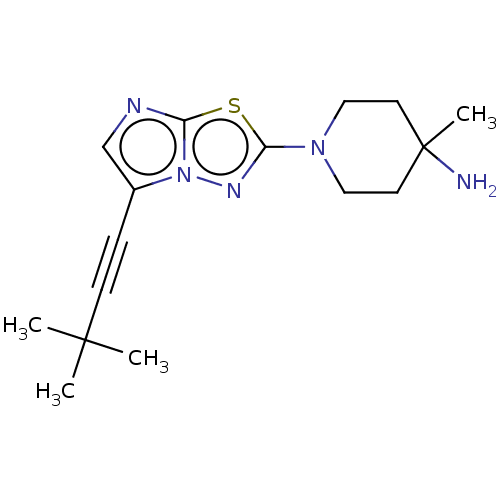
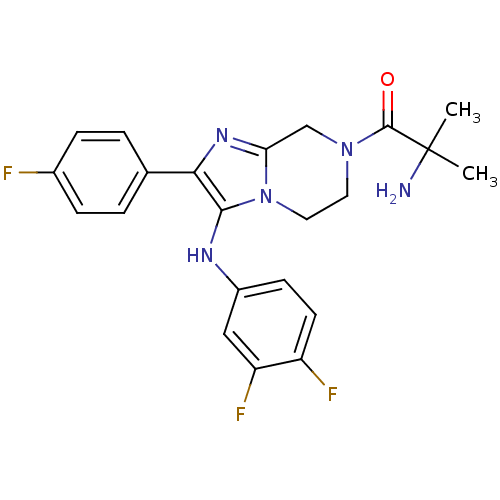
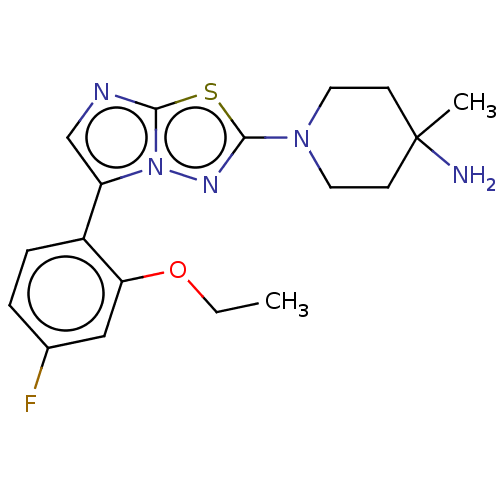
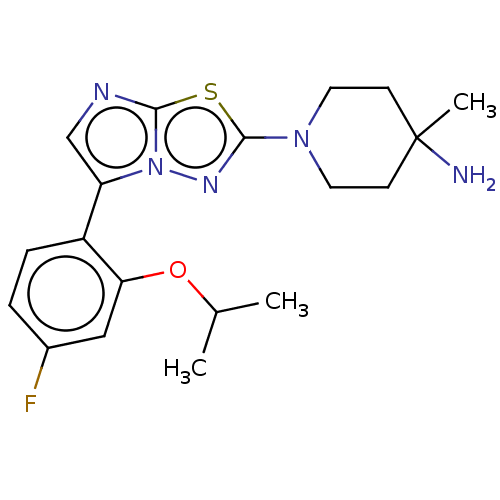
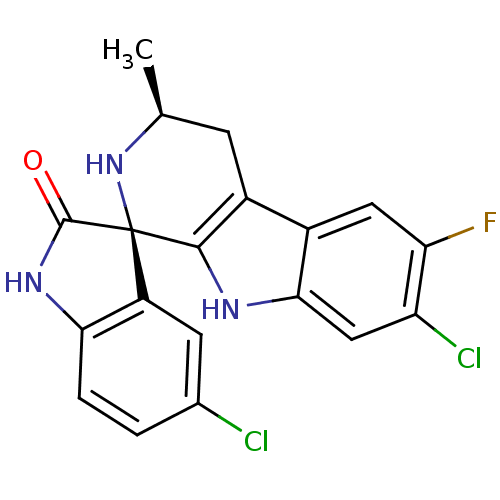
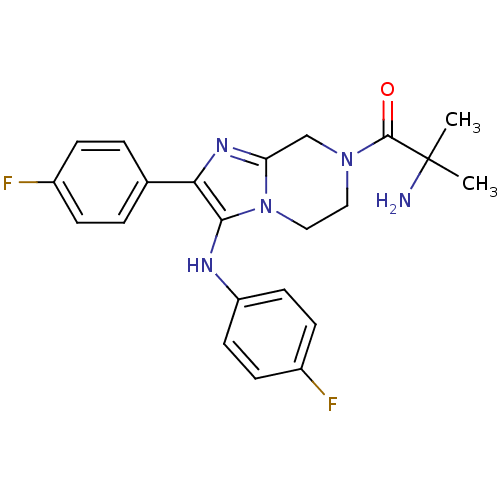
Affinity DataKi: 0.00400nM ΔG°: -66.1kJ/molepH: 4.7 T: 2°CAssay Description:HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P...More data for this Ligand-Target Pair
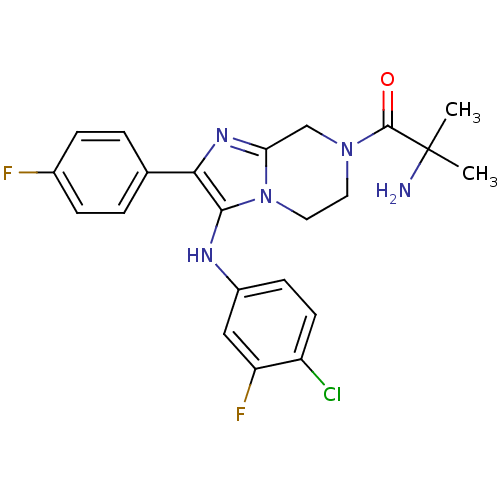
Affinity DataKi: 0.0110nM ΔG°: -63.6kJ/molepH: 4.7 T: 2°CAssay Description:HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P...More data for this Ligand-Target Pair
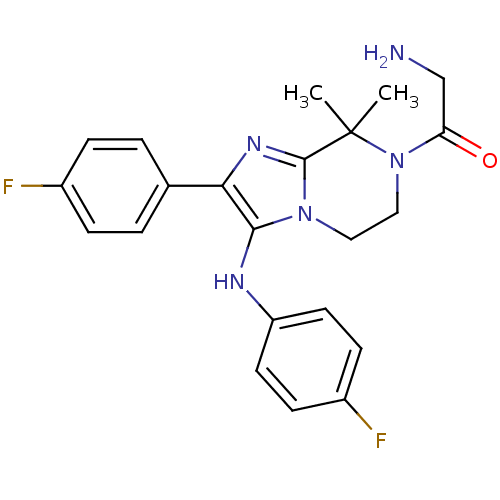
Affinity DataKi: 0.0120nM ΔG°: -63.4kJ/molepH: 4.7 T: 2°CAssay Description:HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P...More data for this Ligand-Target Pair
Affinity DataKi: 0.112nM ΔG°: -57.7kJ/molepH: 4.7 T: 2°CAssay Description:HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P...More data for this Ligand-Target Pair
TargetDihydrofolate reductase(Homo sapiens (Human))
Kenya Medical Research Institute/Wellcome Trust Collaborative Research Program
Curated by ChEMBL
Kenya Medical Research Institute/Wellcome Trust Collaborative Research Program
Curated by ChEMBL
Affinity DataKi: 7.70nMAssay Description:Binding affinity to human recombinant DHFR expressed in Escherichia coli BL21(DE3) by competitive binding assayMore data for this Ligand-Target Pair
TargetDihydrofolate reductase(Homo sapiens (Human))
Kenya Medical Research Institute/Wellcome Trust Collaborative Research Program
Curated by ChEMBL
Kenya Medical Research Institute/Wellcome Trust Collaborative Research Program
Curated by ChEMBL
Affinity DataKi: 10.2nMAssay Description:Binding affinity to human recombinant DHFR expressed in Escherichia coli BL21(DE3) by competitive binding assayMore data for this Ligand-Target Pair
TargetDihydrofolate reductase(Homo sapiens (Human))
Kenya Medical Research Institute/Wellcome Trust Collaborative Research Program
Curated by ChEMBL
Kenya Medical Research Institute/Wellcome Trust Collaborative Research Program
Curated by ChEMBL
Affinity DataKi: 30.8nMAssay Description:Binding affinity to human recombinant DHFR expressed in Escherichia coli BL21(DE3) by competitive binding assayMore data for this Ligand-Target Pair
TargetDihydrofolate reductase(Homo sapiens (Human))
Kenya Medical Research Institute/Wellcome Trust Collaborative Research Program
Curated by ChEMBL
Kenya Medical Research Institute/Wellcome Trust Collaborative Research Program
Curated by ChEMBL
Affinity DataKi: 55.6nMAssay Description:Binding affinity to human recombinant DHFR expressed in Escherichia coli BL21(DE3) by competitive binding assayMore data for this Ligand-Target Pair
TargetOxaloacetate decarboxylase(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...)
University of Maryland Biotechnology Institute
University of Maryland Biotechnology Institute
Affinity DataKi: 4.30E+4nMAssay Description:Competitive inhibition constant determined for PA4872 catalyzed oxaloacetate decarboxylation.More data for this Ligand-Target Pair
TargetOxaloacetate decarboxylase(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...)
University of Maryland Biotechnology Institute
University of Maryland Biotechnology Institute
Affinity DataKi: 4.50E+5nMAssay Description:Competitive inhibition constant determined for PA4872 catalyzed oxaloacetate decarboxylation.More data for this Ligand-Target Pair
TargetOxaloacetate decarboxylase(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...)
University of Maryland Biotechnology Institute
University of Maryland Biotechnology Institute
Affinity DataKi: 5.60E+5nMAssay Description:Competitive inhibition constant determined for PA4872 catalyzed oxaloacetate decarboxylation.More data for this Ligand-Target Pair
TargetOxaloacetate decarboxylase(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...)
University of Maryland Biotechnology Institute
University of Maryland Biotechnology Institute
Affinity DataKi: 1.09E+6nMAssay Description:Competitive inhibition constant determined for PA4872 catalyzed oxaloacetate decarboxylation.More data for this Ligand-Target Pair
TargetOxaloacetate decarboxylase(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...)
University of Maryland Biotechnology Institute
University of Maryland Biotechnology Institute
Affinity DataKi: 3.00E+6nMAssay Description:Competitive inhibition constant determined for PA4872 catalyzed oxaloacetate decarboxylation.More data for this Ligand-Target Pair
TargetOxaloacetate decarboxylase(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...)
University of Maryland Biotechnology Institute
University of Maryland Biotechnology Institute
Affinity DataKi: 3.20E+6nMAssay Description:Competitive inhibition constant determined for PA4872 catalyzed oxaloacetate decarboxylation.More data for this Ligand-Target Pair
TargetOxaloacetate decarboxylase(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...)
University of Maryland Biotechnology Institute
University of Maryland Biotechnology Institute
Affinity DataKi: 6.70E+6nMAssay Description:Competitive inhibition constant determined for PA4872 catalyzed oxaloacetate decarboxylation.More data for this Ligand-Target Pair
TargetOxaloacetate decarboxylase(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...)
University of Maryland Biotechnology Institute
University of Maryland Biotechnology Institute
Affinity DataKi: 7.20E+6nMAssay Description:Competitive inhibition constant determined for PA4872 catalyzed oxaloacetate decarboxylation.More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 32nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 45nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 78nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 140nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 690nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 770nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.28E+3nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.33E+3nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of human ERG expressed in CHO cells by Q-patch assayMore data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Novartis Institute For Tropical Diseases
Curated by ChEMBL
Novartis Institute For Tropical Diseases
Curated by ChEMBL
Affinity DataIC50: 1.51E+3nMAssay Description:Inhibition of CYP2C9 in human liver microsome by LC-MS/MS analysisMore data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Novartis Institute For Tropical Diseases
Curated by ChEMBL
Novartis Institute For Tropical Diseases
Curated by ChEMBL
Affinity DataIC50: 1.72E+3nMAssay Description:Inhibition of CYP2C9 in human liver microsome by LC-MS/MS analysisMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.74E+3nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 2.30E+3nMAssay Description:Inhibition of human ERG expressed in CHO cells by Q-patch assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of hERG by Qpatch-clamp methodMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of human FLT3More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4.04E+3nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Novartis Institute For Tropical Diseases
Curated by ChEMBL
Novartis Institute For Tropical Diseases
Curated by ChEMBL
Affinity DataIC50: 4.11E+3nMAssay Description:Inhibition of CYP2C9 in human liver microsome by LC-MS/MS analysisMore data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Novartis Institute For Tropical Diseases
Curated by ChEMBL
Novartis Institute For Tropical Diseases
Curated by ChEMBL
Affinity DataIC50: 4.50E+3nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 4.80E+3nMAssay Description:Inhibition of human ERG expressed in CHO cells by Q-patch assayMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4.96E+3nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 5.31E+3nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetCytochrome P450 2C19(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 5.40E+3nMAssay Description:Inhibition of CYP2C19 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Novartis Institute For Tropical Diseases
Curated by ChEMBL
Novartis Institute For Tropical Diseases
Curated by ChEMBL
Affinity DataIC50: 5.42E+3nMAssay Description:Inhibition of CYP2C9 in human liver microsome by LC-MS/MS analysisMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase haspin(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 5.50E+3nMAssay Description:Inhibition of human Haspin kinase domain using histone H3 biotin peptide as substrate preincubated with enzyme for 30 mins followed by substrate and ...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 5.60E+3nMAssay Description:Inhibition of human ERG expressed in CHO cells by Q-patch assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 5.60E+3nMAssay Description:Inhibition of human ERG expressed in CHO cells by Q-patch assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 5.60E+3nMAssay Description:Inhibition of human ERG channel expressed in CHO cells by patch clamp assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 5.70E+3nMAssay Description:Inhibition of human ERG expressed in CHO cells by Q-patch assayMore data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: >6.00E+3nMAssay Description:Inhibition of human CYP1A2More data for this Ligand-Target Pair
TargetCytochrome P450 2C19(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >6.00E+3nMAssay Description:Inhibition of human CYP2C19More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)