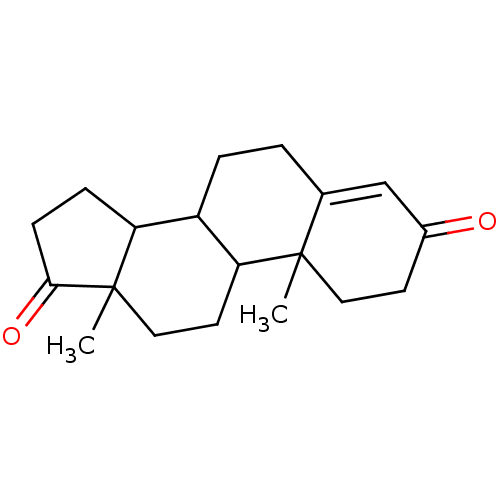
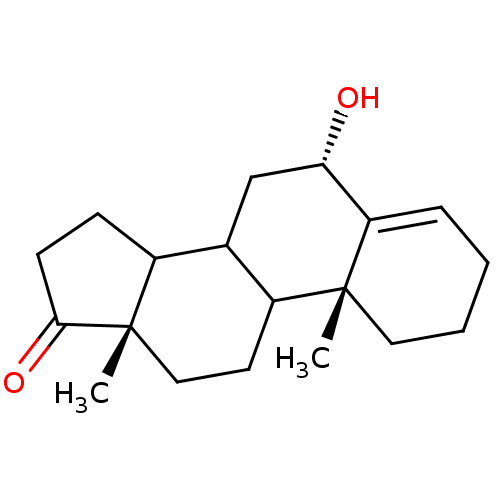
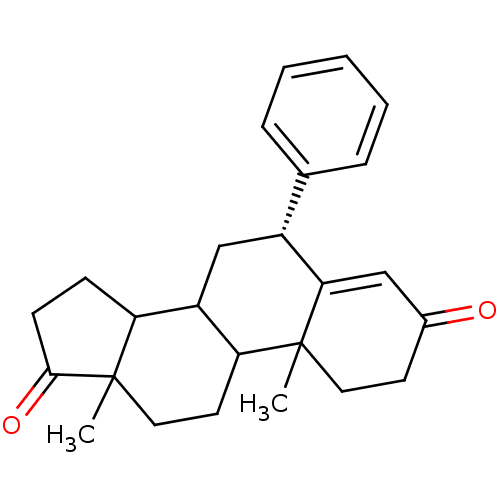
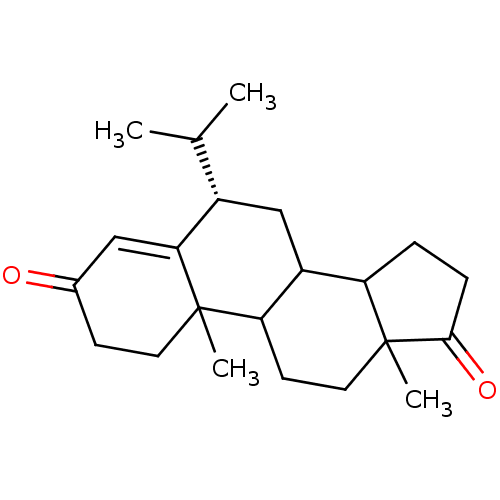
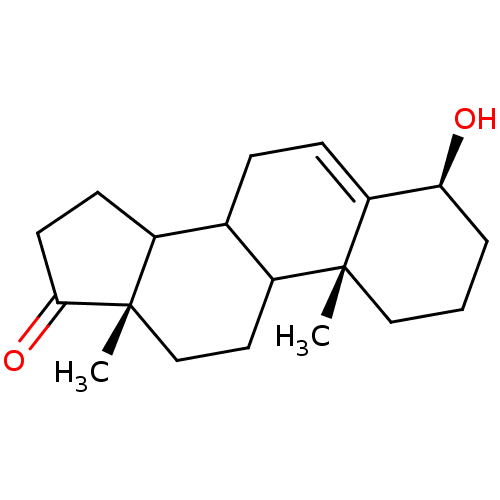
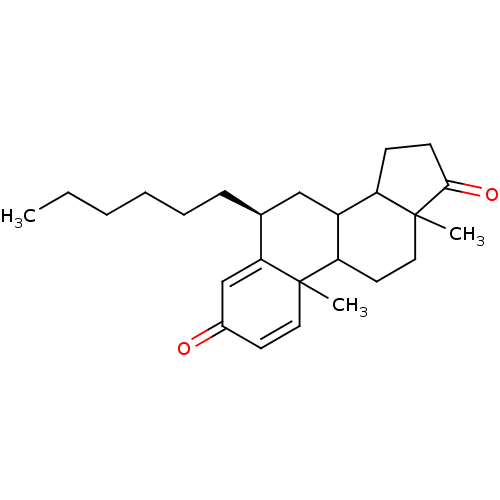
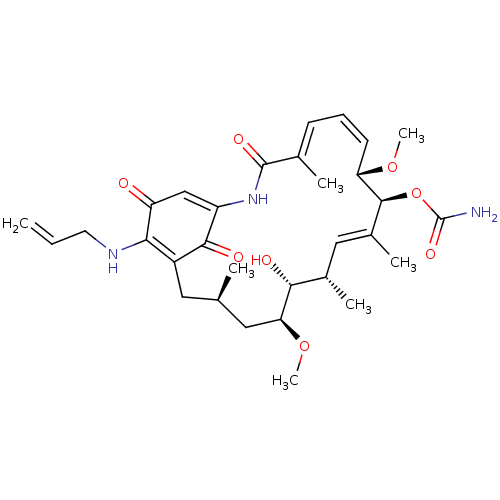
Affinity DataKi: 1.40nMAssay Description:Binding affinity for human placental microsome Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 3.10nM ΔG°: -50.5kJ/mole IC50: 37nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 3.40nM ΔG°: -50.3kJ/mole IC50: 31nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 4.60nMAssay Description:Binding affinity for human placental microsome Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 4.70nM ΔG°: -49.4kJ/mole IC50: 54nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 4.70nMAssay Description:Binding affinity for human placental microsome Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 5nM ΔG°: -49.3kJ/mole IC50: 54nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 5.10nMAssay Description:Binding affinity for human placental microsome aromatase Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 5.30nM ΔG°: -49.1kJ/mole IC50: 49nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 5.60nMAssay Description:Binding affinity for human placental microsome Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 5.80nM ΔG°: -48.9kJ/mole IC50: 49nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 6nM ΔG°: -48.8kJ/mole IC50: 50nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 6nM ΔG°: -48.8kJ/mole IC50: 50nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 6.70nMAssay Description:Binding affinity for human placental microsome aromatase Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 6.80nM ΔG°: -48.5kJ/mole IC50: 60nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 6.80nM IC50: 60nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 7nM ΔG°: -48.4kJ/mole IC50: 60nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 7.80nM ΔG°: -48.1kJ/mole IC50: 60nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 8nM ΔG°: -48.1kJ/mole IC50: 76nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 8.80nMAssay Description:Binding affinity for human placental microsome Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Binding affinity for human placental microsome aromatase Cytochrome P450 19A1More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Yogi Vemana University
Curated by ChEMBL
Yogi Vemana University
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Mixed-type inhibition of electric eel AChE using acetylthiocholine iodide as substrate assessed as free enzyme preincubated for 5 mins followed by su...More data for this Ligand-Target Pair
Affinity DataKi: 11nM ΔG°: -47.3kJ/mole IC50: 140nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Binding affinity for human placental microsome Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 12nM ΔG°: -47.0kJ/mole IC50: 120nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 12nM IC50: 130nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Binding affinity for human placental microsome aromatase Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:The binding affinity was determined on Cytochrome P450 19A1 by analysis of Dixon plotMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Inhibition of 1 uM [1-beta-3H]-androstenedione binding to human placental microsome Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 14nM ΔG°: -46.6kJ/mole IC50: 110nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 14nM ΔG°: -46.6kJ/mole IC50: 180nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
Gifu Pharmaceutical University
Curated by ChEMBL
Gifu Pharmaceutical University
Curated by ChEMBL
Affinity DataKi: 15nMAssay Description:Inhibition of reductase activity of N-terminal 6His-tagged human AKR1B10 expressed in Escherichia coli BL21(DE3) assessed as pyridine-3-aldehyde redu...More data for this Ligand-Target Pair
Affinity DataKi: 16nM ΔG°: -46.3kJ/mole IC50: 200nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 18nM ΔG°: -46.0kJ/mole IC50: 160nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 19nM ΔG°: -45.8kJ/mole IC50: 250nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Yogi Vemana University
Curated by ChEMBL
Yogi Vemana University
Curated by ChEMBL
Affinity DataKi: 19nMAssay Description:Mixed-type inhibition of electric eel AChE using acetylthiocholine iodide as substrate assessed as enzyme-substrate complex preincubated for 5 mins f...More data for this Ligand-Target Pair
Affinity DataKi: 20nM ΔG°: -45.7kJ/mole IC50: 250nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 20nM ΔG°: -45.7kJ/mole IC50: 300nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Binding affinity for aromatase cytochrome P45019A1 by analysis of Dixon plotMore data for this Ligand-Target Pair
Affinity DataKi: 21nM ΔG°: -45.6kJ/mole IC50: 190nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 21nM ΔG°: -45.6kJ/mole IC50: 190nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 21nMAssay Description:Binding affinity for human placental microsome aromatase Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 22nM ΔG°: -45.5kJ/mole IC50: 270nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 22nMAssay Description:Binding affinity for human placental microsome aromatase Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 24nM IC50: 260nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 25nM ΔG°: -45.1kJ/mole IC50: 280nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 25nM ΔG°: -45.1kJ/mole IC50: 280nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 29nMAssay Description:Inhibition of FITC-GA binding to Hsp90alpha (unknown origin) ATPase site after 2 hrs by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataKi: 30nM ΔG°: -44.7kJ/mole IC50: 230nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 31nMAssay Description:Binding affinity for human placental microsome aromatase Cytochrome P450 19A1More data for this Ligand-Target Pair


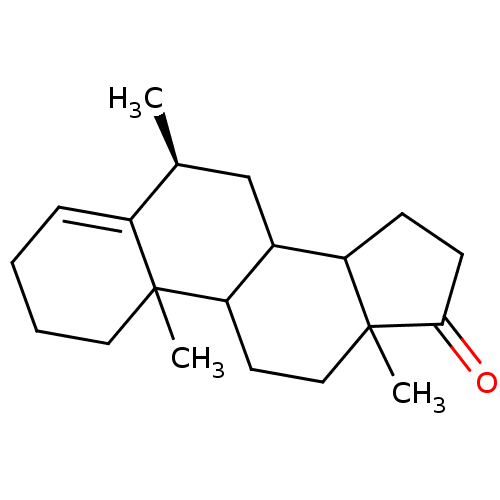



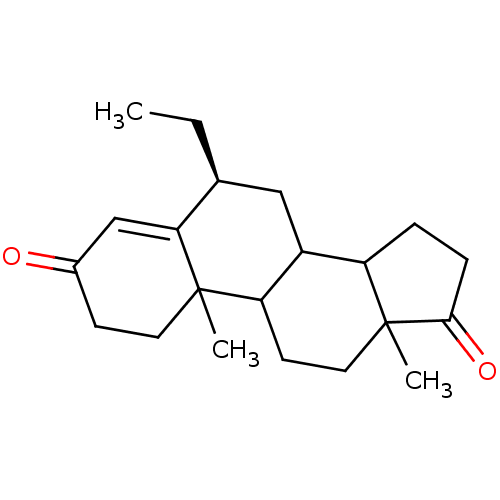

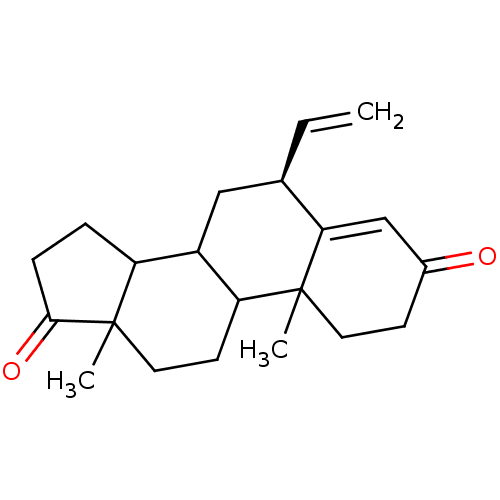
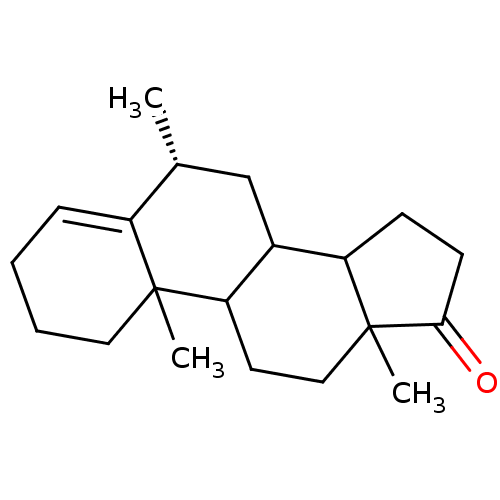



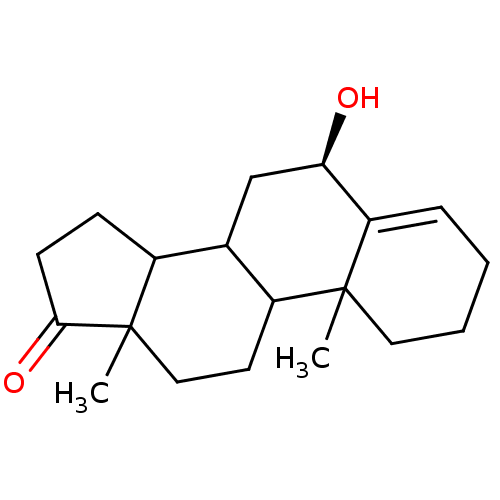
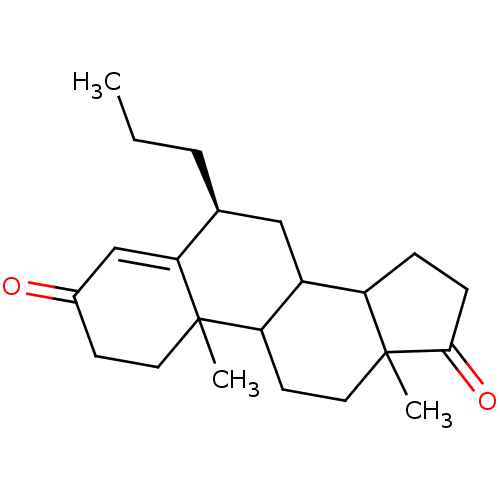
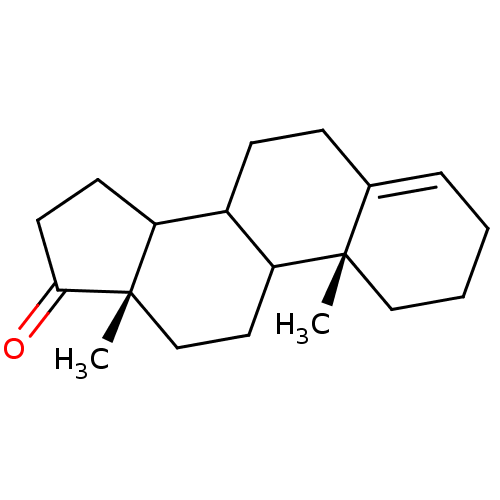






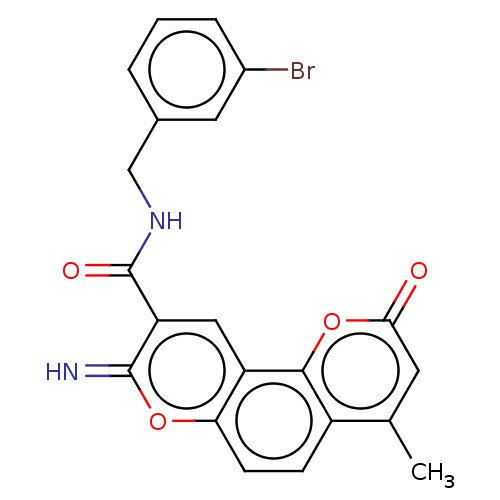

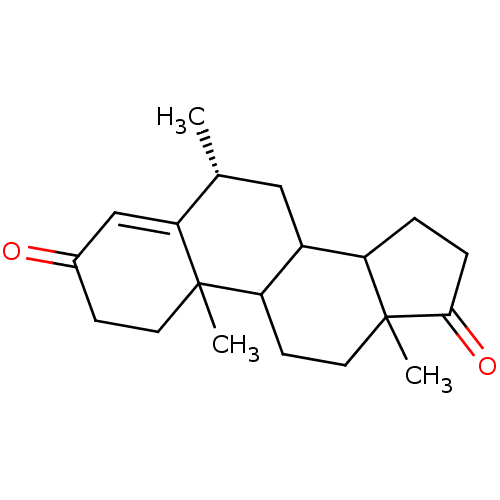
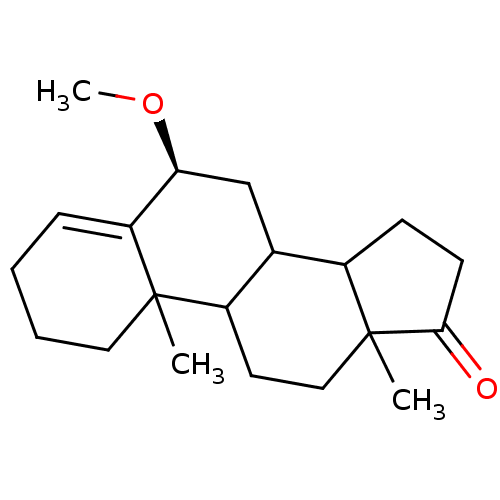


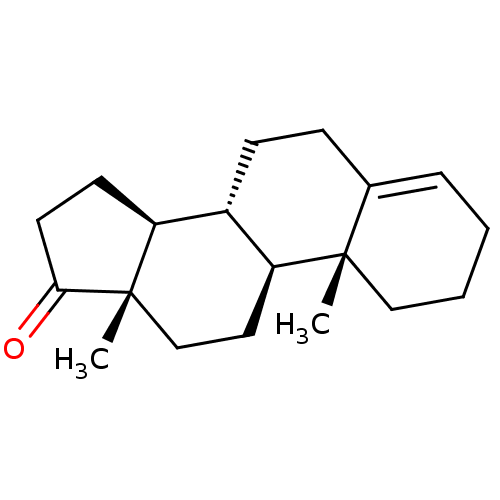



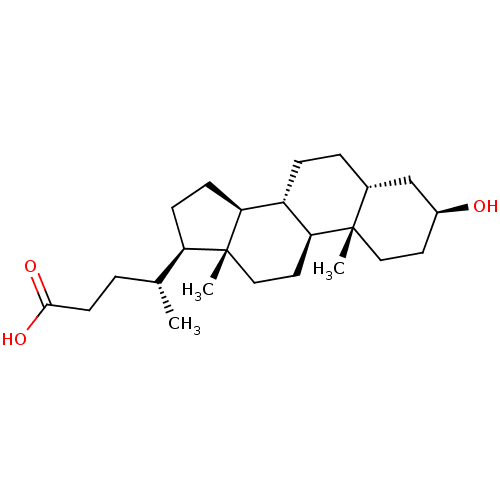

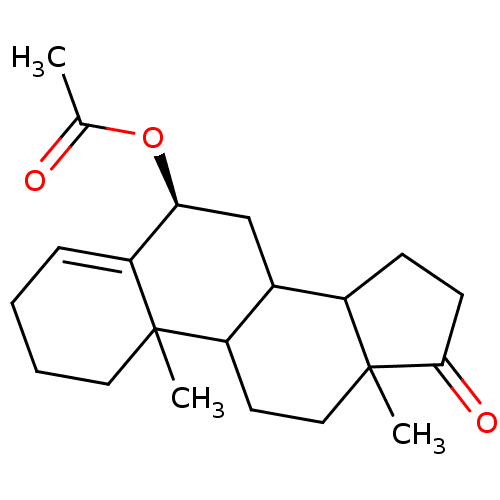


 3D Structure (crystal)
3D Structure (crystal)