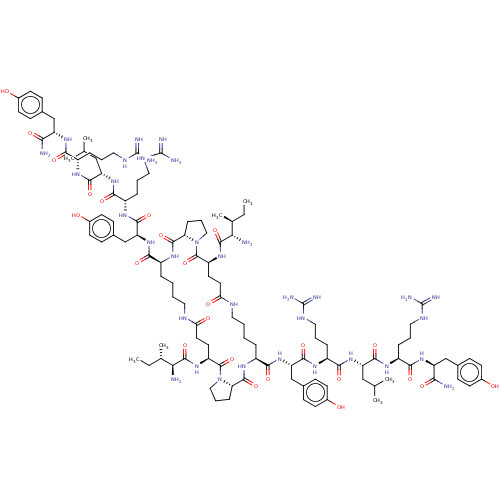
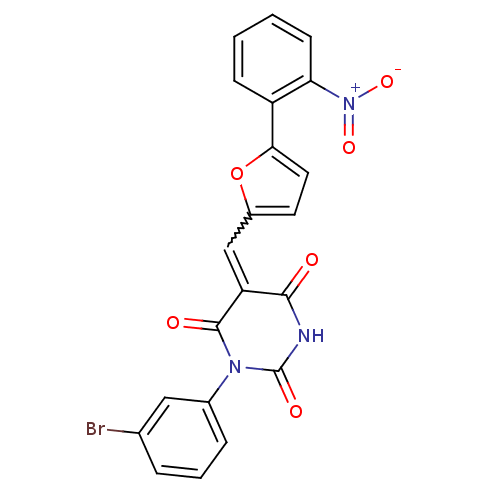
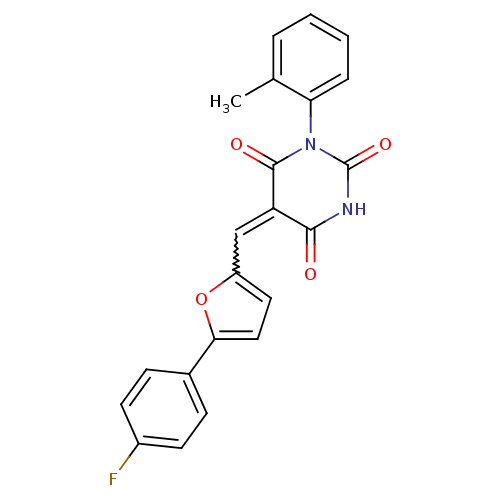
TargetNeuropeptide Y receptor type 1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
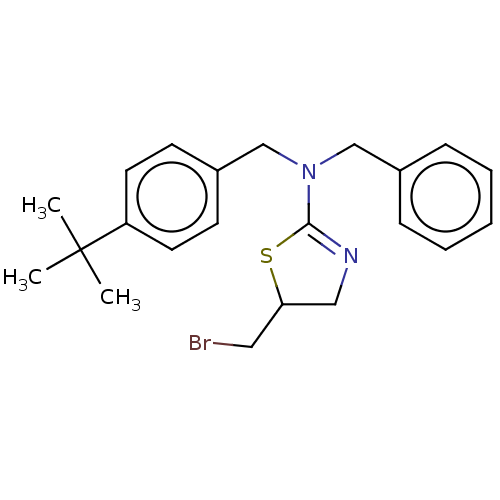
Affinity DataKi: 0.257nMAssay Description:Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y1R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ...More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
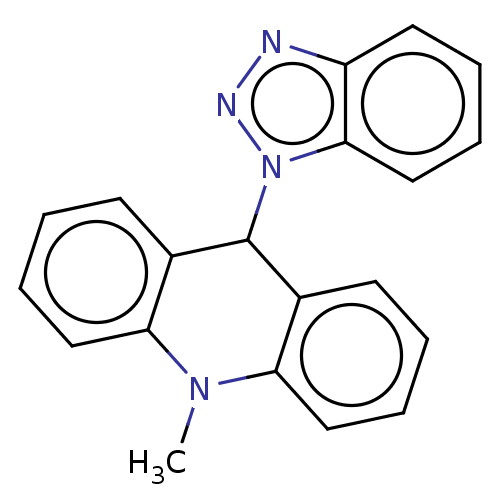
Affinity DataKi: 0.275nMAssay Description:Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y1R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ...More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
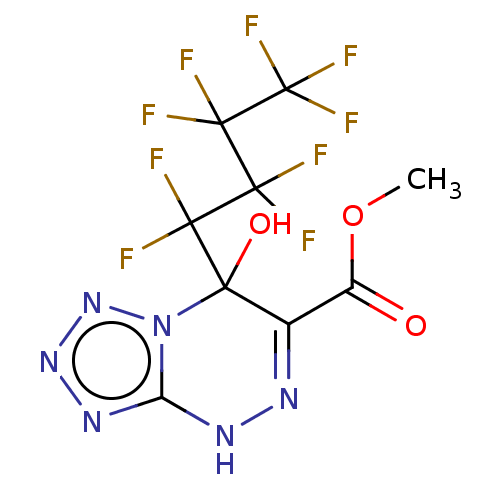
Affinity DataKi: 0.363nMAssay Description:Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y1R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ...More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
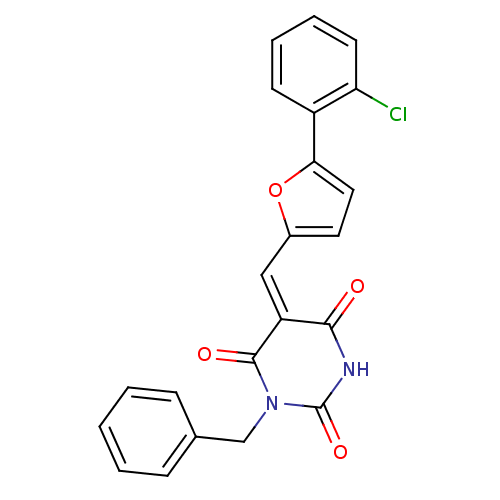
Affinity DataKi: 0.575nMAssay Description:Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y1R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ...More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y1R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ...More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 2.30nMAssay Description:Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y1R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ...More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 4(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 6.5nMAssay Description:Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y4R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ...More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 8.10nMAssay Description:Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y1R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ...More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 13nMAssay Description:Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y1R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ...More data for this Ligand-Target Pair
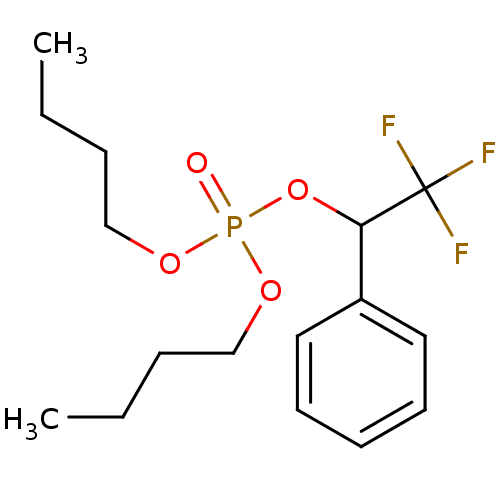
TargetLiver carboxylesterase(Sus scrofa)
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 14nMAssay Description:Competitive inhibition of porcine liver carboxylesterase by double reciprocal Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 17nMAssay Description:Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y1R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ...More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 25nMAssay Description:Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y1R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ...More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 32nMAssay Description:Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y1R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ...More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 4(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 63nMAssay Description:Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y4R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ...More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 4(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 89nMAssay Description:Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y4R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ...More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 4(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 129nMAssay Description:Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y4R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ...More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 4(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 155nMAssay Description:Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y4R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Sus scrofa)
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 156nMAssay Description:Inhibition of pig liver carboxylesterase by Lineweaver-Burk analysisMore data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 4(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 158nMAssay Description:Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y4R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ...More data for this Ligand-Target Pair
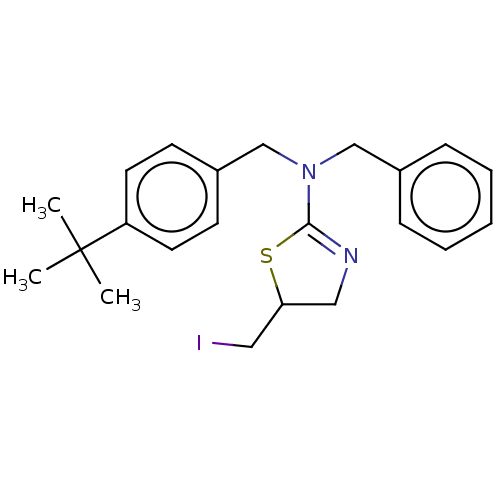
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 160nMAssay Description:Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L...More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 4(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 170nMAssay Description:Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y4R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ...More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 4(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 174nMAssay Description:Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y4R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Sus scrofa)
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 230nMAssay Description:Competitive inhibition of porcine liver carboxylesterase using varying levels of 4-nitrophenol acetate as substrate preincubated for 10 mins followed...More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 4(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 245nMAssay Description:Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y4R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 270nMAssay Description:Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated fo...More data for this Ligand-Target Pair
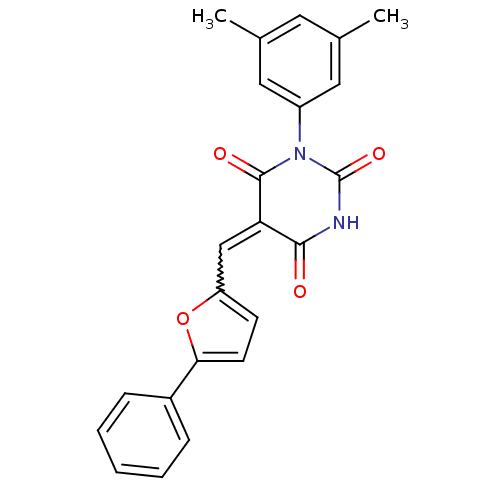
Affinity DataKi: 300nM ΔG°: -38.7kJ/molepH: 7.5 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Sus scrofa)
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 337nMAssay Description:Competitive inhibition of porcine liver carboxylesterase by double reciprocal Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 350nMAssay Description:Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L...More data for this Ligand-Target Pair
Affinity DataKi: 380nM ΔG°: -38.1kJ/molepH: 7.5 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Sus scrofa)
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 400nMAssay Description:Noncompetitive inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate assessed as steady state inhibition constant pre...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 410nMAssay Description:Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Sus scrofa)
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 440nMAssay Description:Noncompetitive inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate assessed as steady state inhibition constant pre...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 490nMAssay Description:Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Sus scrofa)
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 570nMAssay Description:Competitive inhibition of porcine liver carboxylesterase using varying levels of 4-nitrophenol acetate as substrate preincubated for 10 mins followed...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 620nMAssay Description:Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated fo...More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 4(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 631nMAssay Description:Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y4R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 670nMAssay Description:Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated fo...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Sus scrofa)
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 700nMAssay Description:Competitive inhibition of porcine liver carboxylesterase using varying levels of 4-nitrophenol acetate as substrate preincubated for 10 mins followed...More data for this Ligand-Target Pair
Affinity DataKi: 850nM ΔG°: -36.0kJ/molepH: 7.5 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
Affinity DataKi: 880nM ΔG°: -36.0kJ/molepH: 7.5 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
Affinity DataKi: 910nM ΔG°: -35.9kJ/molepH: 7.5 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 4(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 912nMAssay Description:Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y4R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 940nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 1.20E+3nMAssay Description:Noncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 1.34E+3nMAssay Description:Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated fo...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 1.46E+3nMAssay Description:Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 1.53E+3nMAssay Description:Noncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 1.55E+3nMAssay Description:Competitive inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Linewe...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Sus scrofa)
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 1.61E+3nMAssay Description:Competitive inhibition of porcine liver carboxylesterase using varying levels of 4-nitrophenol acetate as substrate preincubated for 10 mins followed...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Institute of Physiologically Active Compounds Russian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 1.79E+3nMAssay Description:Competitive inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Linewe...More data for this Ligand-Target Pair