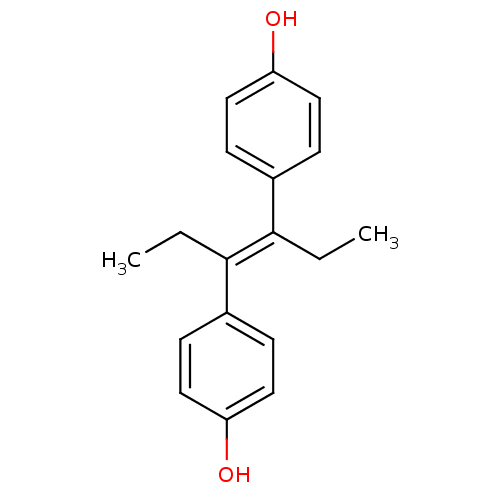
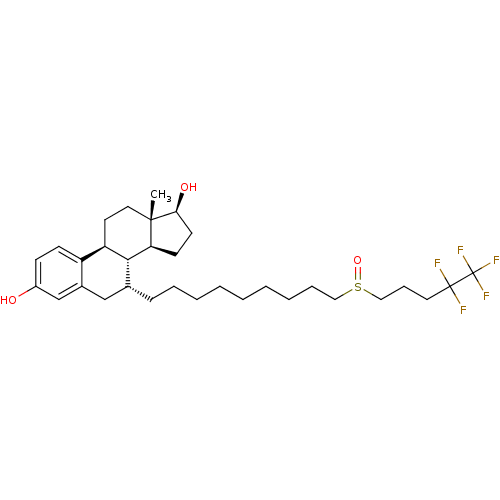
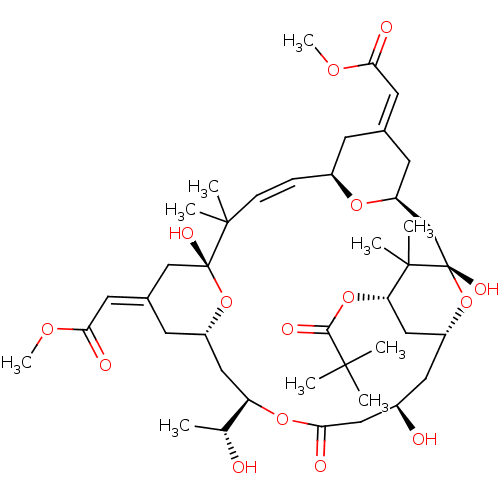
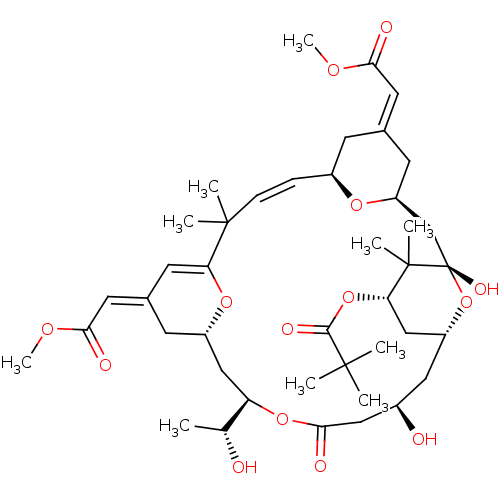
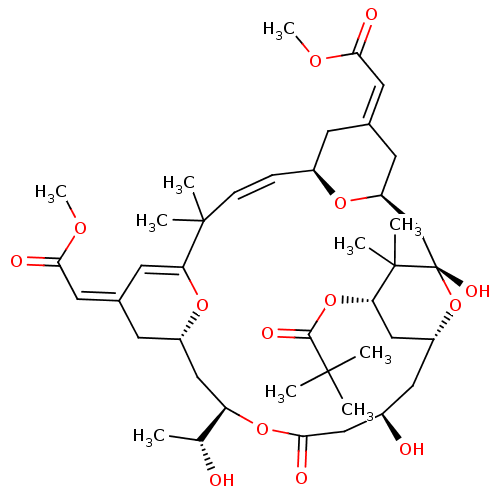
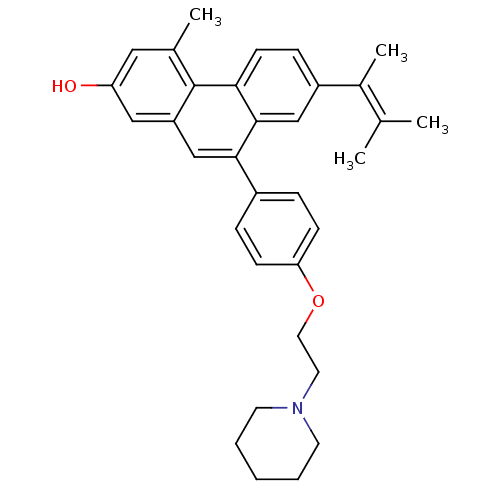
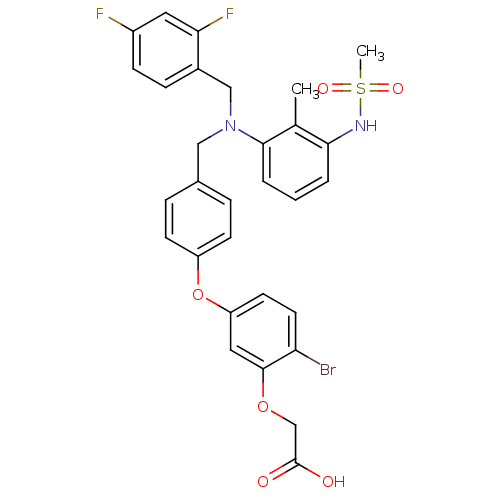
Affinity DataKi: 0.160nMAssay Description:In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor betaMore data for this Ligand-Target Pair
Affinity DataKi: 0.25nMAssay Description:In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataKi: 0.490nMAssay Description:In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataKi: 0.630nMAssay Description:In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor betaMore data for this Ligand-Target Pair
Affinity DataKi: 1.04nMAssay Description:In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataKi: 1.39nMAssay Description:In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor betaMore data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:Displacement of [3H]PDBu form PKCalphaMore data for this Ligand-Target Pair
Affinity DataKi: 4.80nMAssay Description:Displacement of [3H]PDBu form PKCalphaMore data for this Ligand-Target Pair
Affinity DataKi: 8.69nMAssay Description:In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Inhibition of CYP3A4 (unknown origin) assessed as midazolam 1'- hydroxylationMore data for this Ligand-Target Pair
Affinity DataKi: 66nMAssay Description:Inhibition of CYP3A4 (unknown origin) assessed as midazolam 1'- hydroxylationMore data for this Ligand-Target Pair
Affinity DataKi: 118nMAssay Description:Displacement of [3H]PDBu form PKCalphaMore data for this Ligand-Target Pair
Affinity DataKi: 120nMAssay Description:In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataKi: 180nMAssay Description:In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor betaMore data for this Ligand-Target Pair
Affinity DataKi: 188nMAssay Description:Displacement of [3H]PDBu form PKCalphaMore data for this Ligand-Target Pair
Affinity DataKi: 190nMAssay Description:In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor betaMore data for this Ligand-Target Pair
Affinity DataKi: 190nMAssay Description:In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor betaMore data for this Ligand-Target Pair
Affinity DataKi: 200nMAssay Description:In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor betaMore data for this Ligand-Target Pair
Affinity DataKi: 210nMAssay Description:In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataKi: 290nMAssay Description:In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataKi: 490nMAssay Description:In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataKi: 540nMAssay Description:Inhibition of cathepsin D (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 630nMAssay Description:In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor betaMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor betaMore data for this Ligand-Target Pair
Affinity DataKi: >4.00E+3nMAssay Description:Inhibition of renin (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 4.30E+3nMAssay Description:Inhibition of cathepsin D (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: >2.00E+4nMAssay Description:Inhibition of renin (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMpH: 8.0 T: 2°CAssay Description:For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nM EC50: 86nMpH: 8.0 T: 2°CAssay Description:For compounds able to displace the 3(H)-dexamethasone from the receptor an IC50 value (the concentration required to inhibit 50% of the binding of 3(...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 8.0 T: 2°CAssay Description:For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nM EC50: 4.80nMpH: 8.0 T: 2°CAssay Description:For compounds able to displace the 3(H)-dexamethasone from the receptor an IC50 value (the concentration required to inhibit 50% of the binding of 3(...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMpH: 8.0 T: 2°CAssay Description:For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the...More data for this Ligand-Target Pair
Affinity DataIC50: 1.80nM EC50: 330nMpH: 8.0 T: 2°CAssay Description:For compounds able to displace the 3(H)-dexamethasone from the receptor an IC50 value (the concentration required to inhibit 50% of the binding of 3(...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMpH: 8.0 T: 2°CAssay Description:For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMpH: 8.0 T: 2°CAssay Description:For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the...More data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMpH: 8.0 T: 2°CAssay Description:For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the...More data for this Ligand-Target Pair
Affinity DataIC50: 2.40nM EC50: 43nMpH: 8.0 T: 2°CAssay Description:For compounds able to displace the 3(H)-dexamethasone from the receptor an IC50 value (the concentration required to inhibit 50% of the binding of 3(...More data for this Ligand-Target Pair
Affinity DataIC50: 2.60nM EC50: 500nMpH: 8.0 T: 2°CAssay Description:For compounds able to displace the 3(H)-dexamethasone from the receptor an IC50 value (the concentration required to inhibit 50% of the binding of 3(...More data for this Ligand-Target Pair
Affinity DataIC50: 2.90nMpH: 8.0 T: 2°CAssay Description:For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the...More data for this Ligand-Target Pair
Affinity DataIC50: 4.80nM EC50: 440nMpH: 8.0 T: 2°CAssay Description:For compounds able to displace the 3(H)-dexamethasone from the receptor an IC50 value (the concentration required to inhibit 50% of the binding of 3(...More data for this Ligand-Target Pair
Affinity DataIC50: 5.40nM EC50: 255nMpH: 8.0 T: 2°CAssay Description:For compounds able to displace the 3(H)-dexamethasone from the receptor an IC50 value (the concentration required to inhibit 50% of the binding of 3(...More data for this Ligand-Target Pair
Affinity DataIC50: 8.80nMpH: 8.0 T: 2°CAssay Description:For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the...More data for this Ligand-Target Pair
Affinity DataIC50: 120nMpH: 8.0 T: 2°CAssay Description:For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the...More data for this Ligand-Target Pair
Affinity DataIC50: 140nMpH: 8.0 T: 2°CAssay Description:For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the...More data for this Ligand-Target Pair
Affinity DataIC50: 150nMpH: 8.0 T: 2°CAssay Description:For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the...More data for this Ligand-Target Pair
Affinity DataIC50: 180nMpH: 8.0 T: 2°CAssay Description:For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMpH: 8.0 T: 2°CAssay Description:For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the...More data for this Ligand-Target Pair
Affinity DataIC50: 690nMpH: 8.0 T: 2°CAssay Description:For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMpH: 8.0 T: 2°CAssay Description:For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the...More data for this Ligand-Target Pair

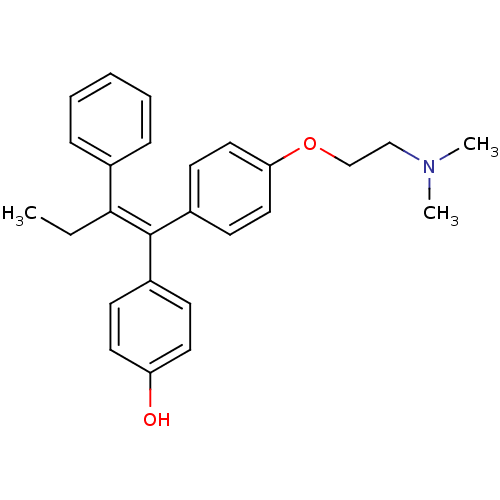
 3D Structure (crystal)
3D Structure (crystal)