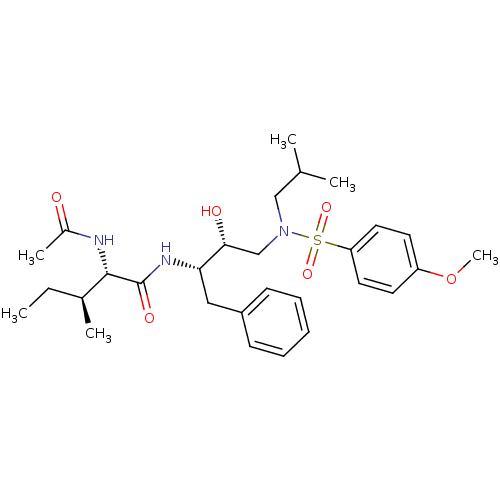
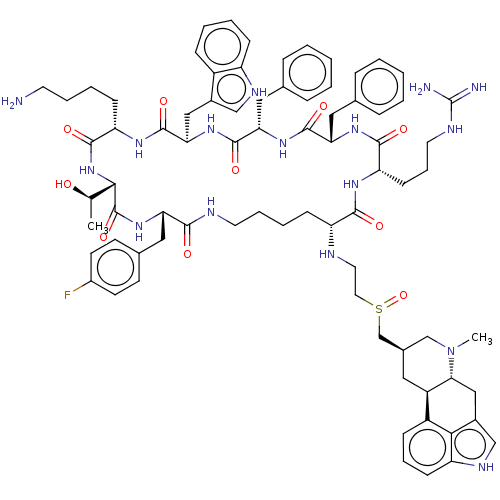
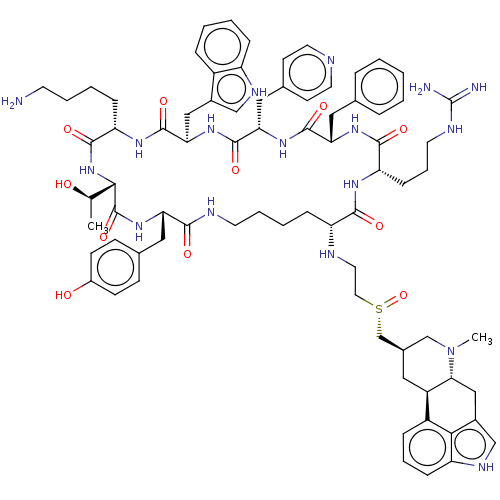
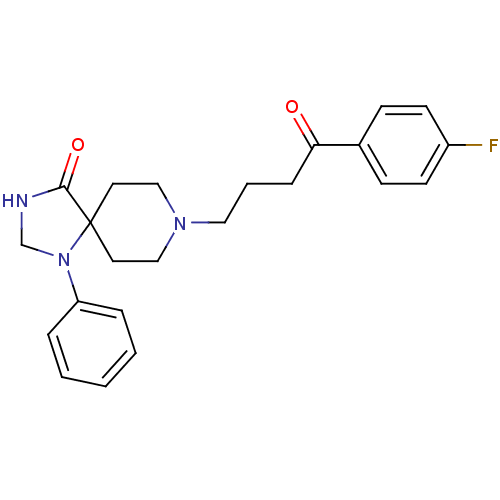
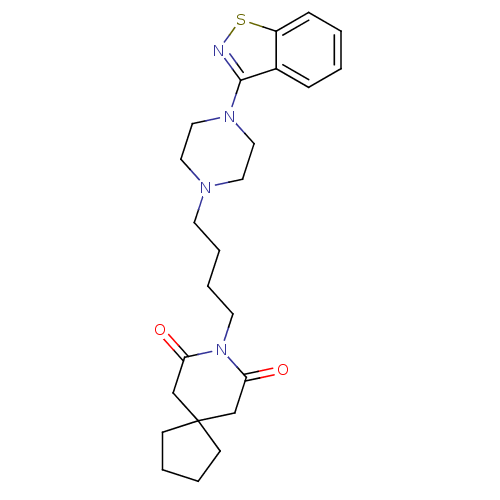
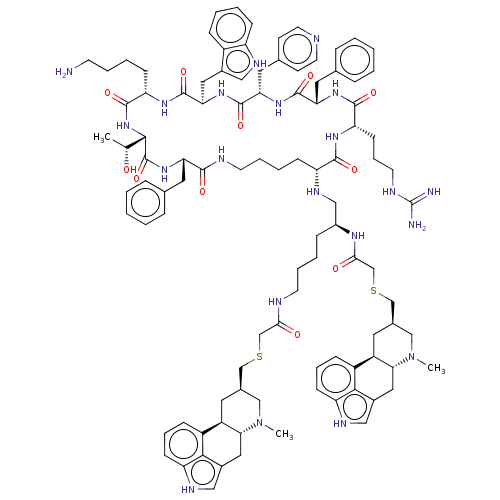
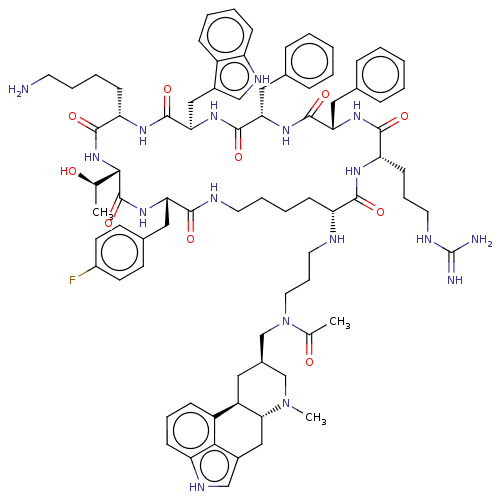
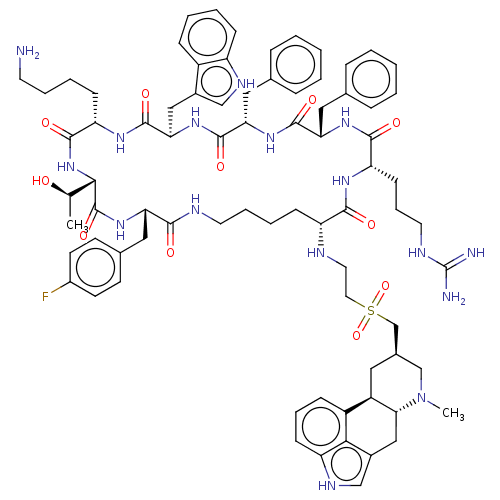
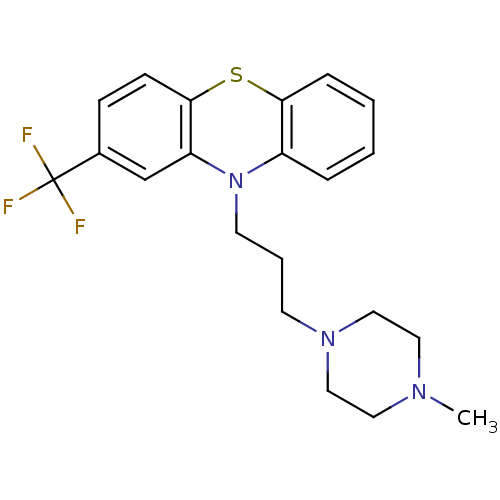
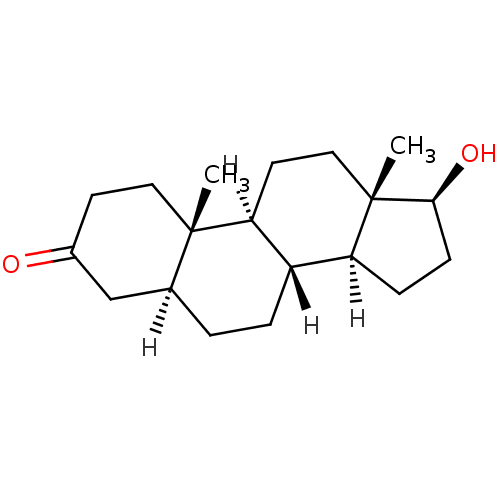
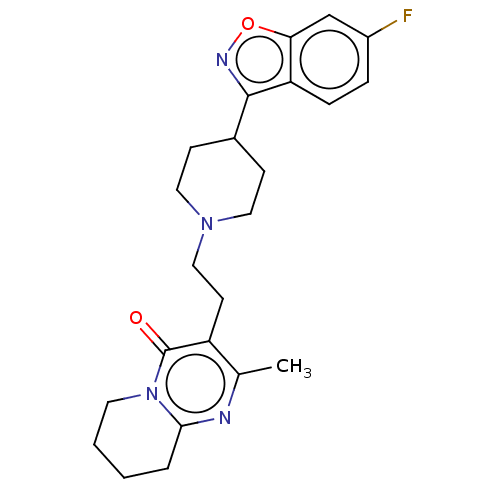
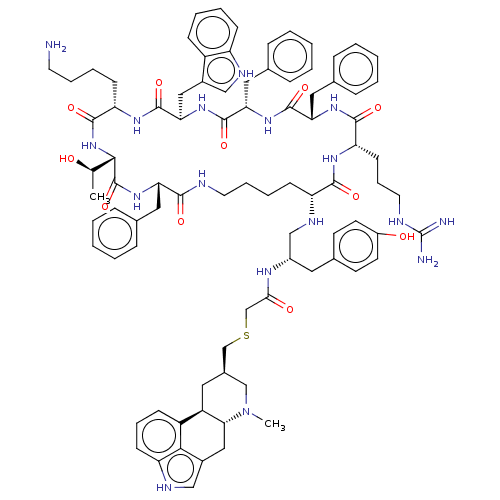
Affinity DataKi: 0.0200nM ΔG°: -61.1kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
Affinity DataKi: 0.0200nM ΔG°: -61.1kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
Affinity DataKi: 0.0200nM ΔG°: -61.1kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
Affinity DataKi: 0.0210nMAssay Description:HIV protease inhibitor activities were determined by fluorescence resonance energy transfer (FRET) method. Protease substrate, Arg-Glu-(EDANS)-Ser-G...More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [484-582,I494L,I497V,V499I,D514N,E519D,R541K,D544E,Q553K,N572D,L573M](Human immunodeficiency virus)
Massachusetts Institute of Technology
Massachusetts Institute of Technology
Affinity DataKi: 0.0280nMAssay Description:HIV protease inhibitor activities were determined by fluorescence resonance energy transfer (FRET) method. Protease substrate, Arg-Glu-(EDANS)-Ser-G...More data for this Ligand-Target Pair
Affinity DataKi: 0.0400nM ΔG°: -59.3kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
Affinity DataKi: 0.0400nM ΔG°: -59.3kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
Affinity DataKi: 0.0400nM ΔG°: -59.3kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
Affinity DataKi: 0.0400nM ΔG°: -59.3kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
Affinity DataKi: 0.0460nMAssay Description:HIV protease inhibitor activities were determined by fluorescence resonance energy transfer (FRET) method. Protease substrate, Arg-Glu-(EDANS)-Ser-G...More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [484-582,I497V,V499I,E519D,R541K,D544E,Q553K,A555V,G557S,I568V,L574M,L573M](Human immunodeficiency virus)
Massachusetts Institute of Technology
Massachusetts Institute of Technology
Affinity DataKi: 0.0480nMAssay Description:HIV protease inhibitor activities were determined by fluorescence resonance energy transfer (FRET) method. Protease substrate, Arg-Glu-(EDANS)-Ser-G...More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [484-582,I494L,I497V,V499I,D514N,E519D,R541K,D544E,Q553K,N572D,L573M](Human immunodeficiency virus)
Massachusetts Institute of Technology
Massachusetts Institute of Technology
Affinity DataKi: 0.0490nMAssay Description:HIV protease inhibitor activities were determined by fluorescence resonance energy transfer (FRET) method. Protease substrate, Arg-Glu-(EDANS)-Ser-G...More data for this Ligand-Target Pair
Affinity DataKi: 0.0500nM ΔG°: -58.8kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
Affinity DataKi: 0.0500nM ΔG°: -58.8kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
Affinity DataKi: 0.0500nM ΔG°: -58.8kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
TargetD(2) dopamine receptor(Rattus norvegicus (rat))
Case Western Reserve University
Curated by PDSP Ki Database
Case Western Reserve University
Curated by PDSP Ki Database
Affinity DataKi: 0.0600nM ΔG°: -58.3kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2A(Rattus norvegicus (rat))
Case Western Reserve University
Curated by PDSP Ki Database
Case Western Reserve University
Curated by PDSP Ki Database
Affinity DataKi: 0.0700nMAssay Description:HIV protease inhibitor activities were determined by fluorescence resonance energy transfer (FRET) method. Protease substrate, Arg-Glu-(EDANS)-Ser-G...More data for this Ligand-Target Pair
Affinity DataKi: 0.0800nM ΔG°: -57.6kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
Affinity DataKi: 0.0800nM ΔG°: -57.6kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
Affinity DataKi: 0.0800nM ΔG°: -57.6kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
Affinity DataKi: 0.0800nM ΔG°: -57.6kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
Affinity DataKi: 0.0900nM ΔG°: -57.3kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
Affinity DataKi: 0.0900nM ΔG°: -57.3kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [484-582,I494L,I497V,V499I,E519D,I534V,R541K,D544E,Q553K,A555V,L573M](Human immunodeficiency virus)
Massachusetts Institute of Technology
Massachusetts Institute of Technology
Affinity DataKi: 0.0920nMAssay Description:HIV protease inhibitor activities were determined by fluorescence resonance energy transfer (FRET) method. Protease substrate, Arg-Glu-(EDANS)-Ser-G...More data for this Ligand-Target Pair
Affinity DataKi: 0.120nM ΔG°: -56.6kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
Affinity DataKi: 0.129nMAssay Description:HIV protease inhibitor activities were determined by fluorescence resonance energy transfer (FRET) method. Protease substrate, Arg-Glu-(EDANS)-Ser-G...More data for this Ligand-Target Pair
Affinity DataKi: 0.130nM ΔG°: -56.4kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
Affinity DataKi: 0.130nM ΔG°: -56.4kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
Affinity DataKi: 0.140nM ΔG°: -56.2kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [484-582,I494L,I497V,V499I,E519D,I534V,R541K,D544E,Q553K,A555V,L573M](Human immunodeficiency virus)
Massachusetts Institute of Technology
Massachusetts Institute of Technology
Affinity DataKi: 0.144nMAssay Description:HIV protease inhibitor activities were determined by fluorescence resonance energy transfer (FRET) method. Protease substrate, Arg-Glu-(EDANS)-Ser-G...More data for this Ligand-Target Pair
Affinity DataKi: 0.146nMAssay Description:HIV protease inhibitor activities were determined by fluorescence resonance energy transfer (FRET) method. Protease substrate, Arg-Glu-(EDANS)-Ser-G...More data for this Ligand-Target Pair
Affinity DataKi: 0.150nM ΔG°: -56.1kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
Affinity DataKi: 0.160nM ΔG°: -55.9kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 7(Rattus norvegicus (rat))
National Institute of Neurological Disorders and Stroke
Curated by PDSP Ki Database
National Institute of Neurological Disorders and Stroke
Curated by PDSP Ki Database
Affinity DataKi: 0.160nM ΔG°: -55.9kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
Affinity DataKi: 0.170nM ΔG°: -55.8kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
Affinity DataKi: 0.170nM ΔG°: -55.8kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
TargetD(2) dopamine receptor(Rattus norvegicus (rat))
Case Western Reserve University
Curated by PDSP Ki Database
Case Western Reserve University
Curated by PDSP Ki Database
Affinity DataKi: 0.194nMAssay Description:HIV protease inhibitor activities were determined by fluorescence resonance energy transfer (FRET) method. Protease substrate, Arg-Glu-(EDANS)-Ser-G...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nM ΔG°: -57.6kJ/mole EC50: 5.10nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Binding affinity to human androgen receptor expressed in monkey COS7 cells by whole cell binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.210nM ΔG°: -55.2kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
Affinity DataKi: 0.230nM ΔG°: -55.0kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
Affinity DataKi: 0.240nM ΔG°: -54.9kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2A(Rattus norvegicus (rat))
Case Western Reserve University
Curated by PDSP Ki Database
Case Western Reserve University
Curated by PDSP Ki Database
Affinity DataKi: 0.25nM ΔG°: -54.8kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
Affinity DataKi: 0.25nM ΔG°: -54.8kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
Affinity DataKi: 0.280nM ΔG°: -54.5kJ/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair

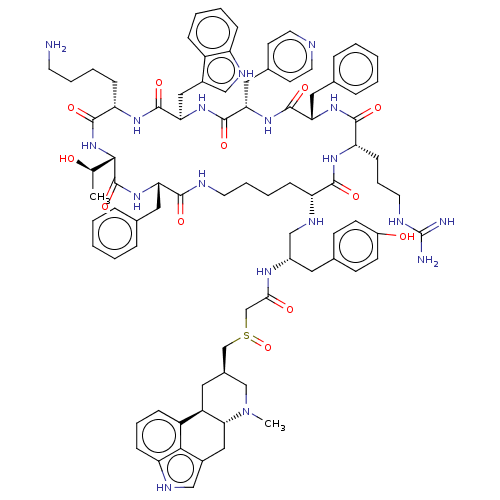
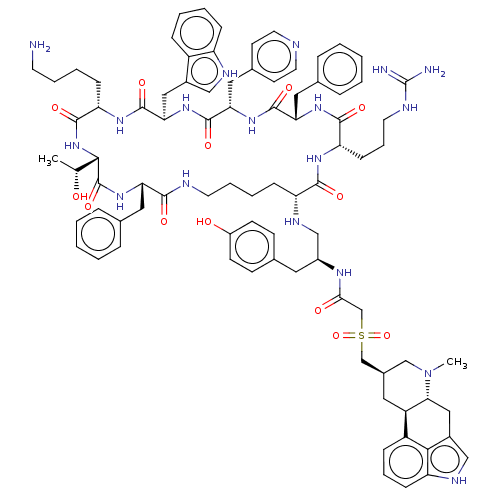
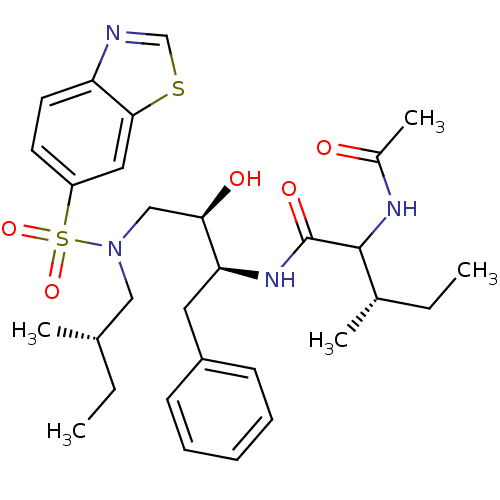
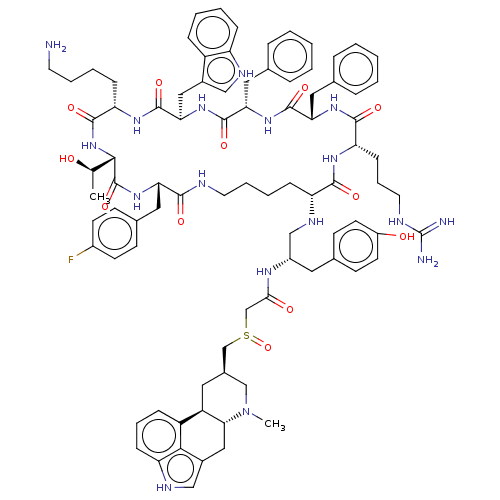

 3D Structure (crystal)
3D Structure (crystal)